Belimumab: Unveiling Its Role in Lupus and Beyond
Quick Facts About Belimumab
What is Belimumab?
Belimumab is a monoclonal antibody used to treat systemic lupus erythematosus (SLE), an autoimmune disease. It is marketed under the brand name Benlysta.
What is the mechanism of action for Belimumab?
Belimumab works by inhibiting the B-lymphocyte stimulator (BLyS), a protein that plays a central role in the survival and activity of B cells, which are involved in autoimmune responses.
What are the clinical applications of Belimumab?
Belimumab is primarily used to treat lupus, including lupus nephritis, by reducing disease activity and flares in patients with systemic lupus erythematosus.
1.) Understanding Belimumab
Belimumab, sold under the brand name Benlysta, is a monoclonal antibody specifically engineered for the treatment of systemic lupus erythematosus (SLE), a complex autoimmune disorder where the immune system mistakenly targets healthy tissues. It functions by targeting and neutralizing B-lymphocyte stimulator (BLyS), a cytokine vital for the survival and activation of B cells. BLyS overexpression in autoimmune conditions like lupus promotes the proliferation and persistence of autoreactive B cells, which produce harmful autoantibodies that drive disease progression.
Belimumab binds to BLyS, preventing it from interacting with its receptors on B cells. This blockade diminishes the survival and activation of autoreactive B cells, thereby reducing autoantibody levels and alleviating the pathological immune response. This mechanism makes Belimumab a pioneering therapy, offering a more targeted approach compared to traditional immunosuppressive treatments that indiscriminately dampen the entire immune system.
Approved in 2011, Belimumab has become an essential treatment option for patients with moderate to severe lupus, especially those with active disease unresponsive to conventional therapies. Clinical studies have consistently shown its efficacy in reducing disease activity, minimizing flare-ups, and lowering the need for corticosteroids. These benefits translate into improved patient quality of life and a reduced risk of long-term organ damage.
Belimumab’s introduction has shifted the paradigm of lupus treatment toward biologic therapies, emphasizing precision medicine. Ongoing research continues to explore its potential in treating other autoimmune disorders, solidifying its role as a cornerstone in advancing therapeutic options for immune-mediated diseases.
2.) Mechanism of Action of Belimumab
Belimumab, a monoclonal antibody, exerts its therapeutic effect by targeting and inhibiting B-lymphocyte stimulator (BLyS), a cytokine crucial for the survival and function of B cells. Under normal physiological conditions, BLyS plays a vital role in regulating B cell maturation, ensuring the proper development of the immune response. However, in autoimmune diseases such as systemic lupus erythematosus (SLE), BLyS is overproduced, leading to an accumulation of autoreactive B cells. These abnormal B cells produce autoantibodies that attack healthy tissues and contribute to the chronic inflammation and tissue damage characteristic of lupus.
Belimumab binds specifically to BLyS, preventing its interaction with receptors on B cells. This blockade disrupts the survival signals for autoreactive B cells, leading to their apoptosis and reducing their numbers in circulation. By curbing the population of autoantibody-producing B cells, Belimumab effectively decreases the levels of circulating autoantibodies, mitigating the immune system’s attack on the body’s tissues.
This targeted mechanism makes Belimumab particularly effective in controlling the hyperactive immune response seen in lupus. Clinical trials and real-world studies have shown that the drug significantly reduces disease activity, decreases the frequency and severity of flare-ups, and lowers the dependency on corticosteroids, thereby reducing the risk of steroid-related side effects.
Additionally, Belimumab has demonstrated efficacy in managing lupus nephritis, a severe and potentially life-threatening kidney complication of lupus. By addressing the root cause of autoimmune activity, Belimumab has become a cornerstone treatment, offering hope to patients with challenging disease manifestations.

3.) Clinical Applications of Belimumab
Belimumab is primarily used for the treatment of systemic lupus erythematosus (SLE), including its complications such as lupus nephritis. In clinical trials, Belimumab has shown effectiveness in reducing disease activity and improving patient outcomes in both adults and pediatric populations with moderate to severe SLE. It is typically administered as an intravenous infusion or subcutaneous injection, depending on patient preferences and clinical circumstances. For patients with lupus nephritis, a severe kidney complication of lupus, Belimumab has demonstrated the ability to reduce proteinuria (excess protein in the urine) and improve kidney function.
The drug is approved for use in combination with other immunosuppressive treatments for lupus patients who do not respond to standard therapies. Studies have shown that Belimumab can help reduce disease flare-ups, improve organ function, and enhance overall quality of life in lupus patients. Its efficacy in treating patients with resistant or difficult-to-treat forms of lupus makes it a valuable therapeutic option for those who have not responded well to other treatments.
Belimumab’s clinical applications extend beyond lupus, with emerging research investigating its potential in treating other autoimmune disorders, such as rheumatoid arthritis and Sjogren’s syndrome. Ongoing clinical trials are exploring its role in combination therapies, potentially expanding its use in autoimmune conditions and enhancing patient outcomes in a variety of settings.
4.) Exploring Biosimilars for Belimumab
What is a Biosimilar?
A biosimilar is a biologic product highly similar to an already approved reference biologic (in this case, Belimumab). Biosimilars offer researchers an alternative that can provide cost-effective, accessible options for the study of Belimumab’s mechanisms and therapeutic potential.
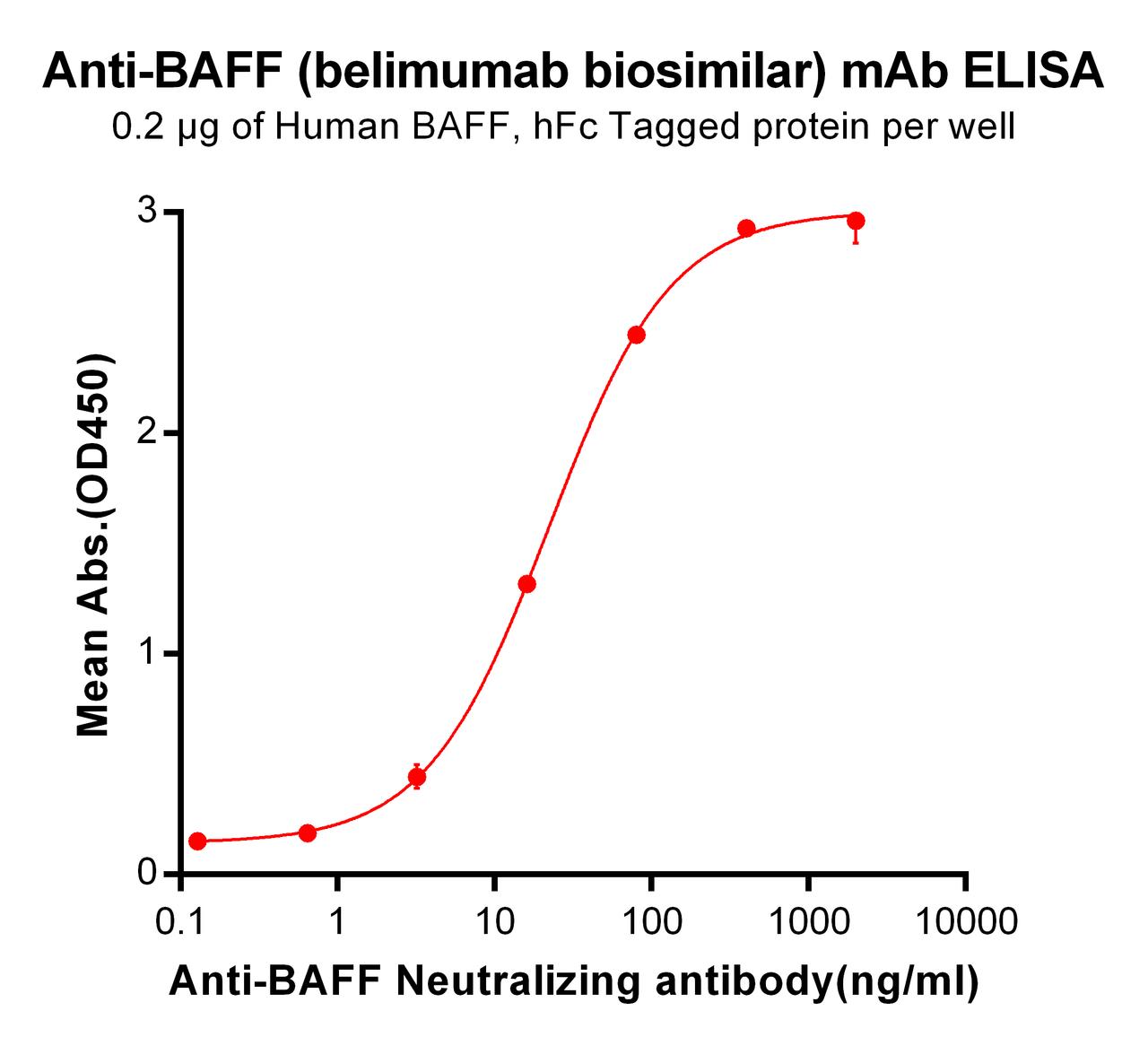
| Belimumab (Anti-BAFF) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | BAFF |
| Reactivity: | Human |
Comparison and Benefits of Belimumab
Belimumab biosimilars maintain the same structure, potency, and mechanism of action as the original drug, ensuring similar therapeutic benefits. However, the production of biosimilars generally offers more flexibility and lower costs, making them attractive for research purposes. These biosimilars are specifically designed for use in ongoing research, helping scientists explore Belimumab’s impact in diverse populations and new indications.
While Belimumab itself has revolutionized treatment for lupus and autoimmune conditions, its biosimilars further facilitate large-scale clinical studies, potentially expanding the scope of its applications in autoimmune disease treatment. Researchers can use biosimilars to test hypotheses, optimize dosages, and explore novel therapeutic combinations without the financial and logistical constraints of the original biologic.
Research Use Only Disclaimer:
It is important to note that these biosimilars are intended for research use only, meaning they are not currently approved for patient treatment but are valuable tools for advancing scientific understanding.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Praluzatamab: Unveiling the Promise of CD47-Targeted Therapy in Cancer Research
Quick Facts About PraluzatamabWhat is Praluzatamab?Praluzatamab is an experimental mon …13th May 2025




