Belantamab: A Game-Changer in Multiple Myeloma Therapy
Quick Facts About Belantamab
What is Belantamab?
Belantamab Mafodotin is a cutting-edge antibody-drug conjugate (ADC) designed for the treatment of relapsed or refractory multiple myeloma.
How does Belantamab work?
It targets B-cell maturation antigen (BCMA) on multiple myeloma cells, delivering a potent cytotoxic agent to destroy cancerous cells effectively.
What are the side effects of Belantamab?
Keratopathy (eye toxicity) is the most notable adverse effect, requiring regular ophthalmic monitoring during treatment.
Is Belantamab approved?
Yes, Belantamab Mafodotin received accelerated approval from the FDA for patients with limited treatment options for multiple myeloma.
1.) Understanding Belantamab
Belantamab Mafodotin (marketed as BLENREP) represents a groundbreaking advancement in the treatment of multiple myeloma, showcasing the potential of antibody-drug conjugates (ADCs) to transform precision oncology. This innovative therapy, developed by GlaxoSmithKline (GSK), uniquely combines the specificity of monoclonal antibodies with the potent cytotoxic effects of a chemotherapeutic payload. By selectively targeting B-cell maturation antigen (BCMA), a protein that is overexpressed on multiple myeloma cells but minimally present on normal tissues, Belantamab maximizes therapeutic efficacy while reducing systemic toxicity. This targeted approach allows for the destruction of cancer cells with improved precision, minimizing collateral damage to healthy cells—a critical advancement in the quest for safer and more effective cancer therapies.
Despite its groundbreaking design and clinical promise, Belantamab has encountered challenges that underscore the complexities of ADC-based therapies. The drug's safety profile necessitates careful patient selection and monitoring, as adverse effects, including ocular toxicity, have been reported. These concerns have prompted the recent withdrawal of BLENREP from some markets, reflecting the need for continued evaluation of its risk-benefit profile. However, this withdrawal does not signify the end of Belantamab's journey but highlights the dynamic nature of drug development. Ongoing research aims to optimize dosing strategies, identify biomarkers for patient selection, and refine ADC technologies, ensuring Belantamab’s potential is fully realized in combating multiple myeloma.
2.) Mechanism of Action of Belantamab
Belantamab Mafodotin operates through a sophisticated and multifaceted mechanism of action, making it a highly effective weapon in the fight against multiple myeloma. Its therapeutic approach is built upon three key components that work in synergy to target and destroy malignant cells while minimizing harm to healthy tissues.
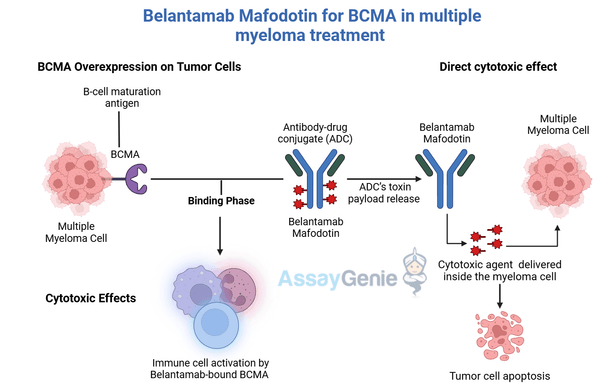
The first component is targeting BCMA, a protein overexpressed on multiple myeloma cells. The monoclonal antibody in Belantamab specifically binds to BCMA, ensuring precise delivery to cancer cells and avoiding off-target effects. This targeted approach serves as the foundation of its precision oncology capabilities.
Secondly, Belantamab delivers a cytotoxic payload directly into the cancer cells. Upon binding to BCMA, the antibody-drug conjugate (ADC) is internalized into the cell, where it releases its cytotoxic agent, monomethyl auristatin F (MMAF). MMAF disrupts microtubule dynamics, which are essential for cell division, ultimately inducing apoptosis in the cancer cells. This efficient delivery system allows for high potency while sparing healthy tissues.
The third component involves immune activation. By binding to BCMA, Belantamab triggers antibody-dependent cellular cytotoxicity (ADCC), engaging the immune system to attack and destroy cancer cells. This additional layer of action amplifies its anti-tumor effects, enhancing overall therapeutic efficacy and reducing collateral damage.
3.) Clinical Applications of Belantamab
Belantamab Mafodotin (Blenrep) is a first-in-class antibody-drug conjugate (ADC) designed to target BCMA (B-cell maturation antigen), a hallmark of malignant plasma cells in multiple myeloma. It is primarily indicated for relapsed or refractory multiple myeloma in patients who have exhausted prior therapeutic options, including immunomodulators, proteasome inhibitors, and anti-CD38 monoclonal antibodies. This innovative therapy leverages its unique mechanism of action to deliver cytotoxic agents directly to myeloma cells, reducing systemic toxicity and enhancing efficacy.
FDA Approval: Belantamab Mafodotin received accelerated approval based on the promising results of the pivotal DREAMM-2 trial. In this study, the drug demonstrated meaningful response rates in heavily pretreated patients, underscoring its potential as a salvage therapy in advanced multiple myeloma.
Emerging Research: Ongoing clinical trials, including DREAMM-7 and DREAMM-8, are investigating Belantamab Mafodotin in combination with established anti-myeloma agents, such as proteasome inhibitors and immunomodulators. These studies aim to enhance efficacy, expand its use across different treatment lines, and establish its role in combination regimens.
Challenges: The primary challenge associated with Belantamab Mafodotin is the risk of keratopathy, a dose-limiting toxicity requiring careful monitoring. To mitigate this risk, the FDA has implemented a Risk Evaluation and Mitigation Strategy ( REMS), restricting its use to certified centers with trained healthcare providers.
Future Directions: Continued research is focused on optimizing dosing schedules, identifying predictive biomarkers for efficacy and toxicity, and exploring alternative administration strategies. These efforts aim to expand the therapeutic potential of Belantamab Mafodotin while addressing safety concerns, ensuring broader access for patients with relapsed or refractory multiple myeloma.
4.) Exploring Biosimilars for Belantamab
What is a Biosimilar?
A biosimilar is a near-identical copy of an original biologic drug, developed to match its safety, efficacy, and quality. These products offer cost-effective alternatives for research and therapeutic use.

| Belantamab (Anti-BCMA) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | BCMA |
| Reactivity: | Human |
How Belantamab Biosimilars Compare
Biosimilars for Belantamab mimic its mechanism of action, targeting BCMA and delivering cytotoxic agents. However, they are designed exclusively for research, enabling scientists to explore new combinations, mechanisms, and applications without regulatory barriers.
Benefits of Biosimilars in Research
Research Use Only Disclaimer
Biosimilars discussed here are intended for research use only and are not approved for clinical applications.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Marina Alberto, PhD
Marina Alberto, PhD, holds a robust academic background in Biotechnology, earning her Bachelor’s Degree and PhD in Science and Technology from Quilmes National University. Her research spans cancer immunotherapy, glycan profiling, and vaccine development, including innovative projects on pediatric leukemia diagnosis and cancer-associated carbohydrate-mimetic vaccines. She currently serves as a Technical Support and Sales Specialist at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
3D Organoid Cell Viability Assay: Assessing Environmental Stress on BENOs for Live Delivery
Ibatici, D. (1), Westhoff, A. (1)Assessing Environmental Stress on BENOs for Live Deli …21st Feb 2025