Basiliximab: Mechanism, Clinical Applications, and Research Advancements
Quick Facts About Basiliximab
What is Basiliximab?
Basiliximab is a monoclonal antibody targeting the CD25 subunit of the IL-2 receptor, primarily used to prevent organ transplant rejection.
What is the mechanism of action for Basiliximab?
It inhibits IL-2 mediated T-cell activation by blocking the CD25 receptor, crucial for immune response regulation.
What are the clinical applications of Basiliximab?
Basiliximab is approved for use in kidney transplantation to reduce the risk of acute rejection and has emerging applications in preventing graft-versus-host disease (GVHD).
1.) Understanding Basiliximab
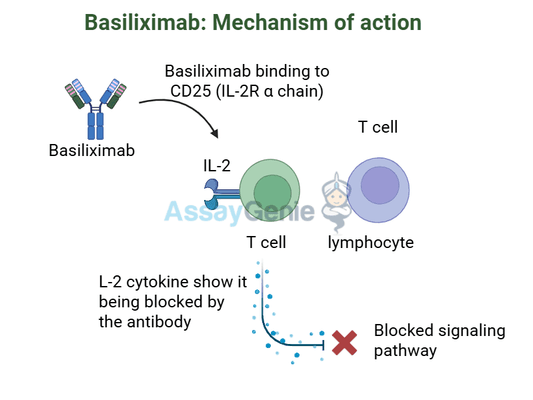
Basiliximab, known commercially as Simulect, is a chimeric monoclonal antibody designed to inhibit immune system responses that lead to organ rejection. It achieves this by specifically targeting the CD25 alpha chain of the interleukin-2 (IL-2) receptor, a critical component in T-cell activation. When T-cells recognize a transplanted organ as foreign, they activate and proliferate through pathways involving the IL-2 receptor. Basiliximab’s precise action blocks this pathway, preventing the immune response that leads to rejection.
Since its FDA approval in 1998, Basiliximab has become a mainstay in transplant medicine, particularly in kidney transplants. It is administered as part of induction therapy, a strategy used to suppress the immune system during the early stages of organ transplantation. By preventing acute rejection, Basiliximab ensures better graft survival and reduces the need for aggressive maintenance immunosuppressive therapies. This selective approach minimizes the side effects commonly associated with broader immunosuppressive agents.
Beyond its established role, Basiliximab is being explored in new therapeutic areas. Researchers are investigating its application in managing graft-versus-host disease (GVHD), a severe complication of hematopoietic stem cell transplants. The drug’s ability to selectively inhibit T-cell activation without depleting these cells entirely is especially advantageous, offering a safer profile compared to other immunosuppressive agents. This growing body of research highlights Basiliximab’s potential to address a range of immune-mediated conditions beyond organ transplantation.
2.) Mechanism of Action of Basiliximab
The mechanism of action of Basiliximab revolves around its ability to selectively block T-cell activation by targeting the IL-2 receptor’s alpha chain, CD25. T-cells play a pivotal role in the immune system’s response to perceived threats, including transplanted organs. When activated, T-cells express CD25 as part of the IL-2 receptor complex, which is essential for their proliferation and survival. Basiliximab binds with high affinity to CD25, effectively blocking the IL-2 signaling pathway. This targeted inhibition prevents the clonal expansion of T-cells, which is a critical step in mounting an immune response against a transplant.
This specificity offers several advantages. Unlike traditional immunosuppressive agents that broadly suppress the immune system, Basiliximab’s targeted approach reduces the risk of opportunistic infections and malignancies. Additionally, its non-depleting nature means it temporarily inhibits T-cell activation without destroying these cells, preserving the immune system’s baseline functionality.
Pharmacokinetically, Basiliximab is designed for sustained action. With a long half-life of approximately 7 days, it provides continuous receptor blockade with just two doses administered within the first four days post-transplant. This dosing regimen ensures consistent therapeutic levels during the critical early period when the risk of rejection is highest.
Recent research is also examining the broader implications of IL-2 pathway inhibition. Basiliximab’s mechanism is being studied in autoimmune diseases and GVHD, where selective modulation of T-cell activity could offer significant therapeutic benefits. This growing interest underscores the versatility and potential of Basiliximab in immunomodulation.
3.) Clinical Applications of Basiliximab
Basiliximab is primarily recognized for its role in kidney transplantation, where it serves as a key component of induction therapy. Induction therapy involves administering immunosuppressive drugs at the time of transplantation to reduce the risk of acute rejection. In kidney transplant recipients, acute rejection can severely compromise graft function and long-term survival. Basiliximab’s ability to selectively inhibit IL-2 mediated T-cell activation makes it particularly effective in preventing this early immune response.
One of the drug’s standout features is its non-depleting nature. Unlike agents that broadly suppress or destroy immune cells, Basiliximab temporarily blocks T-cell activation without depleting the T-cell population. This selective approach reduces the likelihood of side effects such as severe infections or malignancies, which are common with other immunosuppressants. Additionally, the simplified dosing schedule—typically two doses administered shortly after transplantation—enhances patient compliance and minimizes the burden on healthcare providers.
Beyond kidney transplantation, Basiliximab is being explored for other clinical applications. In the context of hematopoietic stem cell transplantation, early studies suggest that it may help prevent or mitigate graft-versus-host disease (GVHD). GVHD occurs when transplanted donor cells attack the recipient’s tissues, leading to severe complications. By modulating T-cell activity, Basiliximab shows promise in reducing the incidence and severity of GVHD while maintaining graft function.
Emerging research also points to potential uses in autoimmune diseases, where excessive T-cell activation drives pathology. While these applications are still under investigation, they highlight Basiliximab’s versatility and potential to address a broader range of immunological challenges. This evolving therapeutic landscape underscores its importance in both clinical and research settings.
4.) Exploring Biosimilars for Basiliximab
Biosimilars of Basiliximab provide a cost-effective alternative for research and development. These highly similar biological products maintain the safety, efficacy, and quality of the original drug, making them invaluable tools in advancing scientific discovery.
What is a Biosimilar?
A biosimilar is a biologic product that demonstrates no clinically meaningful differences from its reference product in terms of safety, purity, or potency. Biosimilars offer a more accessible option for research institutions and pharmaceutical companies, fostering innovation and discovery.
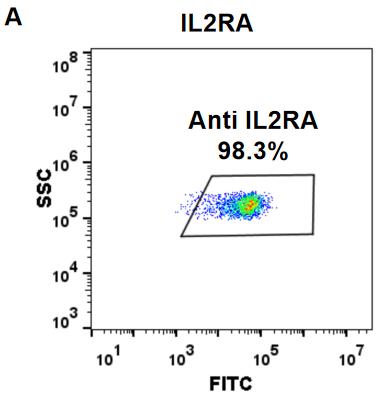
| Basiliximab Reference Biosimilar Antibody (Anti-IL2RA) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | IL-2RA |
| Reactivity: | Human |
How Does the Basiliximab Biosimilar Compare?
Basiliximab biosimilars mirror the original drug’s ability to inhibit CD25-mediated T-cell activation. While they share the same mechanism of action, biosimilars are often more affordable and accessible, enabling broader research applications.
Benefits for Research:
- Cost-Effectiveness: Reduced production costs translate to lower prices, increasing accessibility for researchers.
- Enhanced Research: Availability of biosimilars supports preclinical and clinical studies exploring new indications and combinations.
- Consistency: Rigorous manufacturing standards ensure high-quality and reliable performance.
Research Use Only Disclaimer:
Basiliximab biosimilars are designated for research purposes and are not intended for clinical use. Their role is to advance understanding of IL-2 signaling and its therapeutic implications.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Praluzatamab: Unveiling the Promise of CD47-Targeted Therapy in Cancer Research
Quick Facts About PraluzatamabWhat is Praluzatamab?Praluzatamab is an experimental mon …13th May 2025