Anifrolumab: Transforming Lupus Treatment with Targeted Immunotherapy
Quick Facts About Anifrolumab
What is Anifrolumab?
Anifrolumab is a monoclonal antibody that targets the type I interferon receptor, playing a crucial role in treating systemic lupus erythematosus (SLE).
What is the mechanism of action for Anifrolumab?
Anifrolumab blocks type I interferon signaling, reducing inflammation and immune overactivation associated with lupus.
What are the clinical applications of Anifrolumab?
It is primarily approved for treating moderate to severe systemic lupus erythematosus (SLE) in adults who do not respond to standard therapy.
1.) Understanding Anifrolumab
Anifrolumab is a promising breakthrough in the field of immunotherapy, particularly for the treatment of autoimmune diseases, with a specific focus on systemic lupus erythematosus (SLE). Developed by AstraZeneca under the brand name Saphnelo, Anifrolumab is one of the first targeted therapies aimed at modulating the immune system’s overactive responses in lupus patients. SLE is a complex autoimmune condition where the immune system mistakenly attacks healthy tissues, causing widespread inflammation and organ damage. One of the key drivers of this process is the overactivation of the type I interferon (IFN) pathway, which occurs in approximately 60-80% of lupus patients. Traditional lupus treatments, such as corticosteroids and immunosuppressive agents, are often not sufficiently effective and come with significant side effects, including the suppression of the immune system, which can lead to infections, weight gain, and long-term damage to bones and other tissues. Anifrolumab, however, offers a more targeted and specific approach to treating lupus by inhibiting the type I interferon receptor.
This approach significantly reduces the underlying inflammation without broadly suppressing immune function, offering a more precise therapeutic solution Anifrolumab’s development marks a significant step forward in treating autoimmune conditions, as it addresses the root cause of the disease rather than simply managing symptoms. By reducing disease activity, Anifrolumab has the potential to transform the management of lupus and provide a safer, more effective option for patients who have previously struggled to find relief from conventional treatments.
2.) Mechanism of Action of Anifrolumab
Anifrolumab works by targeting the type I interferon receptor, a key player in the immune dysregulation seen in systemic lupus erythematosus (SLE). The type I interferon pathway is critical in lupus, as it promotes an exaggerated immune response that attacks healthy tissues. In patients with lupus, type I interferons are produced in excessive amounts and stimulate immune cells to attack the body’s organs and tissues, resulting in widespread inflammation. Anifrolumab is a monoclonal antibody that specifically binds to the interferon-alpha receptor, preventing the activation of the interferon signaling pathway. By blocking this interaction, Anifrolumab effectively dampens the overactive immune responses associated with lupus, reducing disease activity and preventing flare-ups.
This action is particularly important in lupus, where type I interferons are involved in many of the disease’s hallmark symptoms, including skin rashes, joint pain, and kidney inflammation. In clinical studies, including the pivotal TULIP-1 and TULIP-2 trials, Anifrolumab has demonstrated the ability to significantly reduce disease activity in lupus patients, improving both physical and quality-of-life outcomes.
It has also shown to reduce the need for corticosteroids, which are typically used to manage inflammation but come with a wide range of long-term side effects. By targeting a specific inflammatory pathway, Anifrolumab offers a more precise and effective treatment strategy for lupus compared to traditional immunosuppressive therapies. This selective action also means that Anifrolumab may be able to reduce the risk of complications such as infections and organ damage, which are often associated with broader immune suppression.
3.) Clinical Applications of Anifrolumab
Anifrolumab has been approved for the treatment of moderate to severe systemic lupus erythematosus (SLE), particularly in patients who have not responded well to conventional therapies. One of the primary advantages of Anifrolumab over traditional treatments is its ability to target the root cause of the disease rather than broadly suppressing the immune system. Traditional lupus therapies, such as corticosteroids, immunosuppressants, and antimalarials, aim to reduce inflammation but often do so at the cost of general immune suppression, which can leave patients vulnerable to infections and other complications. In contrast, Anifrolumab specifically inhibits the type I interferon pathway, which is known to drive the inflammatory process in lupus, without broadly suppressing immune function.
This precision therapy helps reduce flare-ups and can significantly lower the need for corticosteroids, thus minimizing their long-term side effects. Clinical studies have shown that Anifrolumab can improve disease control, reduce the frequency of flare-ups, and enhance patients' quality of life by mitigating the debilitating symptoms of lupus. Beyond lupus, there is growing interest in exploring Anifrolumab’s potential in treating other autoimmune diseases, such as dermatomyositis and systemic sclerosis. Research is ongoing to determine whether Anifrolumab can provide similar benefits for patients with these conditions, as they also exhibit elevated type I interferon levels. Early-stage trials are promising, suggesting that Anifrolumab could become a broader tool in the treatment of autoimmune disorders. The precision of Anifrolumab’s action makes it an exciting and potentially transformative addition to the immunotherapy landscape, offering a safer and more effective treatment alternative for patients with autoimmune diseases.
4.) Exploring Biosimilars for Anifrolumab
What is a Biosimilar?
Biosimilars are highly similar versions of biologic drugs, developed to provide cost-effective alternatives while maintaining comparable efficacy and safety. They play a crucial role in research and therapeutic innovation by improving access to advanced biologics.
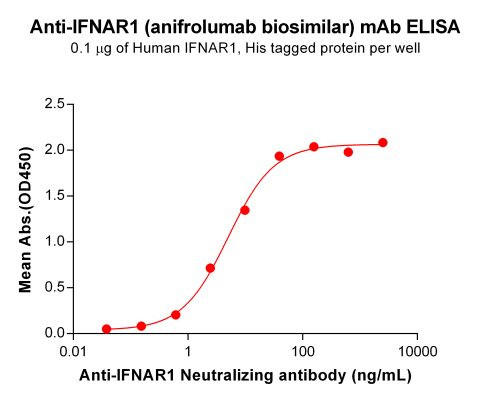
| Anifrolumab (Anti-IFNAR1) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | IFNAR1 |
| Reactivity: | Human |
How Does an Anifrolumab Biosimilar Compare?
Biosimilars of Anifrolumab are being developed to expand research applications and explore new treatment strategies. While they are not identical to the original drug, they maintain the same mechanism of action, making them valuable tools for studying type I interferon inhibition in autoimmune diseases.
Benefits of Anifrolumab Biosimilars
- Enhanced Research Opportunities: Researchers can use biosimilars to investigate novel treatment combinations.
- Improved Accessibility: Biosimilars reduce costs, enabling broader clinical and preclinical exploration.
- Advancing Drug Development: They facilitate innovation in targeted autoimmune therapies.
Research Use Only Disclaimer:
Anifrolumab biosimilars are intended for research purposes only and are not approved for clinical use in patients.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By David Lee, PhD
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



