Alsevalimab: Targeting CD47 for Cancer Research and Treatment
Quick Facts About Alsevalimab
What is Alsevalimab?
Alsevalimab is a monoclonal antibody targeting CD47, a protein involved in immune evasion by cancer cells. By blocking CD47, Alsevalimab enhances the immune system's ability to recognize and attack tumors.
What is the mechanism of action for Alsevalimab?
Alsevalimab binds to CD47, preventing its interaction with SIRPα receptors on macrophages. This disrupts the "don't eat me" signal that helps tumors evade immune detection, thereby promoting anti-tumor immune responses.
What are the clinical applications of Alsevalimab?
Alsevalimab is being explored in clinical trials for various cancers, including solid tumors and hematologic malignancies, to enhance immune system activity and improve treatment outcomes.
1.) Understanding Alsevalimab
Alsevalimab is a promising new monoclonal antibody that targets the CD47 protein, often referred to as the "don't eat me" signal. This protein is commonly found on the surface of cancer cells, and its presence allows these cells to evade detection by the immune system. Normally, CD47 sends a signal to immune cells, particularly macrophages, instructing them not to engulf and destroy the tumor cells. This mechanism is one of the ways that cancer cells avoid immune surveillance and continue to grow unchecked.
By targeting and blocking CD47, Alsevalimab works to interrupt this evasion tactic, enabling the immune system to recognize and eliminate the cancer cells. In particular, the antibody enhances the function of macrophages, which are a type of immune cell responsible for detecting and destroying foreign invaders, including cancer cells. This mechanism makes Alsevalimab an exciting potential treatment in the field of cancer immunotherapy, as it aims to use the body's own immune system to fight tumors more effectively.
Alsevalimab is being tested in a wide range of cancers, from solid tumors such as lung and breast cancer, to hematologic malignancies like lymphoma and leukemia. Researchers are eager to evaluate its effectiveness, especially in combination with other therapies. Early-phase studies suggest that Alsevalimab could provide a breakthrough for patients whose cancers have been resistant to conventional treatments, such as chemotherapy and radiation. Although more data is needed, Alsevalimab’s ability to enhance the body’s immune response to cancer could ultimately improve patient outcomes and lead to significant advances in cancer care.
2.) Mechanism of Action of Alsevalimab
The fundamental action of Alsevalimab revolves around its ability to target the CD47 receptor, which is found on the surface of many cancer cells. Under normal circumstances, CD47 sends a "don't eat me" signal to immune cells, especially macrophages, which are a key component of the body’s innate immune response. This signal prevents macrophages from recognizing and engulfing cancer cells, allowing tumors to grow and spread without being detected by the immune system.
Alsevalimab works by binding directly to CD47, blocking its interaction with SIRPα, a receptor found on the surface of macrophages. This blockade lifts the inhibitory signal that cancer cells rely on to evade immune detection. By disrupting this immune checkpoint, Alsevalimab allows macrophages to recognize and "eat" cancer cells, thereby enhancing the body's natural immune response. This immune modulation is central to its function as an immune checkpoint inhibitor.
In preclinical and early-phase clinical trials, this novel mechanism has shown considerable promise, particularly in combination with other immunotherapies. Researchers are exploring how Alsevalimab can improve the effectiveness of established cancer treatments, such as checkpoint inhibitors, which work by blocking other immune checkpoint proteins like PD-1 or CTLA-4. By combining therapies that target different aspects of immune suppression in the tumor microenvironment, Alsevalimab could significantly boost the immune system’s ability to fight cancer. Its unique mechanism positions it as a potential game-changer in cancer immunotherapy, with the possibility of improving outcomes for patients with a variety of difficult-to-treat cancers.
3.) Clinical Applications of Alsevalimab
Alsevalimab is currently being investigated in clinical trials for its potential to treat a broad spectrum of cancers, ranging from solid tumors to hematologic malignancies. The antibody’s ability to target CD47 has made it a valuable candidate in the field of immuno-oncology, as it directly influences the immune system’s capacity to recognize and attack cancer cells. Unlike traditional cancer treatments, which often rely on chemotherapy or radiation, Alsevalimab aims to harness and enhance the body’s own immune defenses, providing a more targeted and potentially less toxic treatment approach.
In clinical trials, Alsevalimab is often combined with other immune therapies, such as checkpoint inhibitors and monoclonal antibodies that target other immune pathways. Early results have shown promising outcomes, particularly in patients with refractory or relapsed cancers that have not responded to conventional treatments. For example, some trials have demonstrated that Alsevalimab can help improve immune response and increase treatment efficacy in cancers that express high levels of CD47, a protein that facilitates immune evasion.
The potential of Alsevalimab as a treatment for advanced or resistant cancers is especially exciting. If ongoing trials confirm its safety and efficacy, it could become a new option for patients with limited treatment choices. By improving immune system activity, Alsevalimab may allow for better survival rates, particularly in cancers that are resistant to standard therapies. This approach also holds promise for reducing the reliance on traditional chemotherapies, which often come with significant side effects. If proven effective, Alsevalimab could represent a significant advancement in cancer immunotherapy, offering hope to patients with otherwise difficult-to-treat conditions.
4.) Advancing Research on Alsevalimab Biosimilars
What is a Biosimilar?
A biosimilar is a biologic medical product that is highly similar to an already approved reference product, such as Alsevalimab, in terms of its structure, mechanism of action, and clinical effect. Biosimilars are not identical to the original product but are close enough to be interchangeable in terms of efficacy and safety, offering a more affordable treatment option for research purposes.
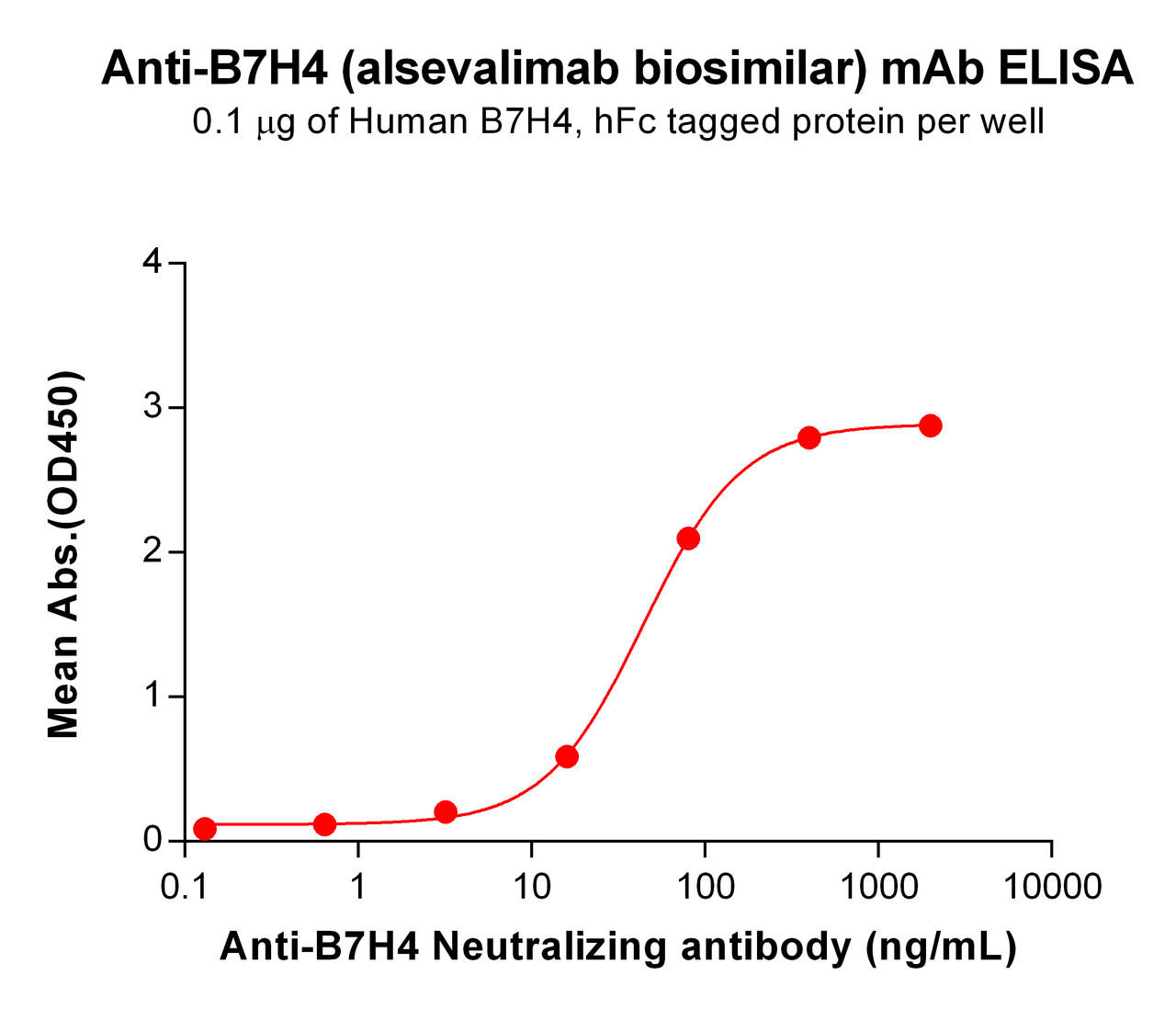
| Alsevalimab (Anti-B7-H4) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | B7-H4 |
| Reactivity: | Human |
How Alsevalimab Biosimilar Compares to Alsevalimab
Alsevalimab biosimilars have emerged as valuable tools in advancing research by providing researchers with an affordable, reliable alternative to the original monoclonal antibody. These biosimilars mimic the function and activity of Alsevalimab, allowing for studies that can explore the drug’s broader applications without the cost burden of the original product. While Alsevalimab biosimilars are used in research and development, they are not yet approved for clinical use outside of this context.
Benefits of Alsevalimab Biosimilars
Research Use Only Disclaimer:
It’s essential to remember that Alsevalimab biosimilars are for research use only. While they offer promising potential for exploring the drug’s capabilities in various settings, they are not approved for direct clinical use at this stage. The ongoing research and data gathered from these biosimilars will play a crucial role in shaping the future of cancer treatment.
Discover Our Biosimilar Range
At Assay Genie, we specialize in providing high-quality biosimilars for research use! Check out our full biosimilar range to learn more.

By Chris McNally, PhD
Chris McNally, PhD, has a strong foundation in Biomedical Science, completing a PhD scholarship in collaboration with Randox Laboratories and Ulster University. Chris has published extensively in prostate cancer research, focusing on biomarker discovery, cancer risk stratification, and molecular mechanisms such as hypoxia-induced regulation. He currently serves as a Business Development Manager at Assay Genie.
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Validation of MycoGenie Rapid Mycoplasma Detection Kit - A highly sensitive visual determination method for Mycoplasma detection.
The MycoGenie Rapid Mycoplasma Detection Kit enables the detection of 28 Mycoplasma sp …3rd Mar 2025



