B7-H4: A New Frontier in Immune Checkpoint Inhibition
The recent advances in cancer immunotherapy have brought new opportunities to harness the body’s immune system against tumors. Among these advancements is the targeting of immune checkpoints, which play a significant role in immune suppression within the tumor microenvironment. A relatively new target of interest is B7-H4, a member of the B7 family of immune-regulatory proteins. Emerging research indicates that blocking B7-H4 with antibodies such as MIH43 may offer promising therapeutic benefits in various cancers. This article explores the role of B7-H4, its biological functions, and how anti-B7-H4 therapies may improve cancer treatment outcomes.
Overview of Immune Checkpoints and Their Importance in Cancer
Immune checkpoints are molecules that regulate the immune response, ensuring that it remains balanced and does not overreact to non-harmful elements. In cancer, these checkpoints can be exploited by tumors to evade immune detection, allowing them to proliferate unchecked. By inhibiting certain immune checkpoint proteins, it is possible to unleash a patient’s T cells to attack and destroy tumor cells.
Key Immune Checkpoints | Function | Therapeutic Target |
|---|---|---|
Inhibits T-cell activation; dampens immune response | ||
Downregulates T-cell response early in activation | ||
Suppresses T-cell proliferation and cytokine production | MIH43 (Anti-B7-H4 antibody) |
What is B7-H4?
B7-H4, also known as VTCN1 (V-set domain-containing T-cell activation inhibitor 1), is a surface protein belonging to the B7 family. This family is essential in modulating immune responses, either activating or inhibiting immune cells. Unlike other B7 family members, B7-H4 is primarily recognized for its inhibitory functions on T-cell activity, making it a compelling target for immune checkpoint blockade therapy.
B7-H4 is highly expressed in various cancers, including:
- Ovarian cancer
- Breast cancer
- Renal cell carcinoma
- Non-small cell lung cancer (NSCLC)
In normal tissues, B7-H4 expression is relatively low, but it is overexpressed in certain tumors, where it plays a significant role in promoting immune evasion by suppressing T-cell proliferation and cytokine secretion. This makes it an attractive therapeutic target for cancer immunotherapy, similar to other immune checkpoints like PD-1 and CTLA-4.
Mechanism of Action of B7-H4
B7-H4 inhibits the immune system by interacting with receptors on T cells to suppress their activity. This suppression limits the ability of T cells to proliferate and produce cytokines necessary for mounting an effective anti-tumor response. Tumor cells that overexpress B7-H4 use this mechanism to create an immunosuppressive microenvironment, thereby evading immune surveillance.
Key Points on B7-H4 Mechanism
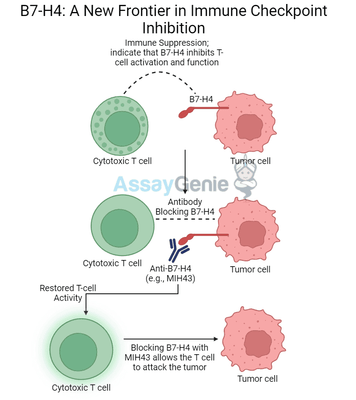
Anti-B7-H4 Antibodies: MIH43 and Its Therapeutic Potential
Antibodies targeting B7-H4, such as MIH43, are designed to block the interaction between B7-H4 and its receptors on T cells. By doing so, they prevent the immunosuppressive effects of B7-H4, allowing T cells to regain their proliferative and cytokine-secreting abilities. This leads to an enhanced anti-tumor immune response, which can result in improved cancer control or eradication.
How Anti-B7-H4 Works
Current Research and Clinical Implications
Preclinical studies and early clinical trials indicate that anti-B7-H4 antibodies, like MIH43, could provide a new avenue for treating cancers that are resistant to existing therapies such as anti-PD-1 or anti-CTLA-4. Since B7-H4 is overexpressed in a variety of tumors, it holds potential for a broad application in oncology.
Key Findings from Preclinical Studies
Tumors Overexpressing B7-H4 | Potential Benefit of Anti-B7-H4 Therapy |
Ovarian cancer | Enhanced immune response and tumor reduction |
Breast cancer | Improved T-cell activation and cytokine production |
Renal cell carcinoma | Synergistic effects with other immune checkpoint blockers |
Non-small cell lung cancer | Targeted immune response and increased survival rates |
Future Directions and Challenges
While anti-B7-H4 therapy holds great promise, several challenges remain:
- Patient selection: Identifying which patients are most likely to benefit from anti-B7-H4 therapy is critical for maximizing its therapeutic impact.
- Combination therapies: Research into combining anti-B7-H4 antibodies with other immune checkpoint inhibitors or chemotherapy is ongoing to determine the most effective treatment regimens.
- Biomarker development: Reliable biomarkers are needed to predict patient responses to anti-B7-H4 treatment and to monitor the effectiveness of therapy over time.
Conclusion
B7-H4 represents an exciting new target in the field of immune checkpoint inhibition, offering a novel approach to restoring immune function in cancer patients. With antibodies like MIH43 showing promising results in early studies, B7-H4 blockade could become a valuable tool in the treatment of cancers that are resistant to other forms of immunotherapy. Continued research and clinical trials will help to determine the full potential of anti-B7-H4 therapies and their role in future cancer treatment strategies.
References
Recent Posts
-
Enavatuzumab: Revolutionizing Cancer Research Through Novel Therapeutics
Quick Facts About EnavatuzumabWhat is Enavatuzumab?Enavatuzumab is a monoclonal antibo …17th Dec 2025 -
Alemtuzumab: Mechanism, Applications, and Biosimilar Advancements
Quick Facts About AlemtuzumabWhat is Alemtuzumab?Alemtuzumab is a monoclonal antibody …17th Dec 2025 -
Praluzatamab: Unveiling the Promise of CD47-Targeted Therapy in Cancer Research
Quick Facts About PraluzatamabWhat is Praluzatamab?Praluzatamab is an experimental mon …13th May 2025