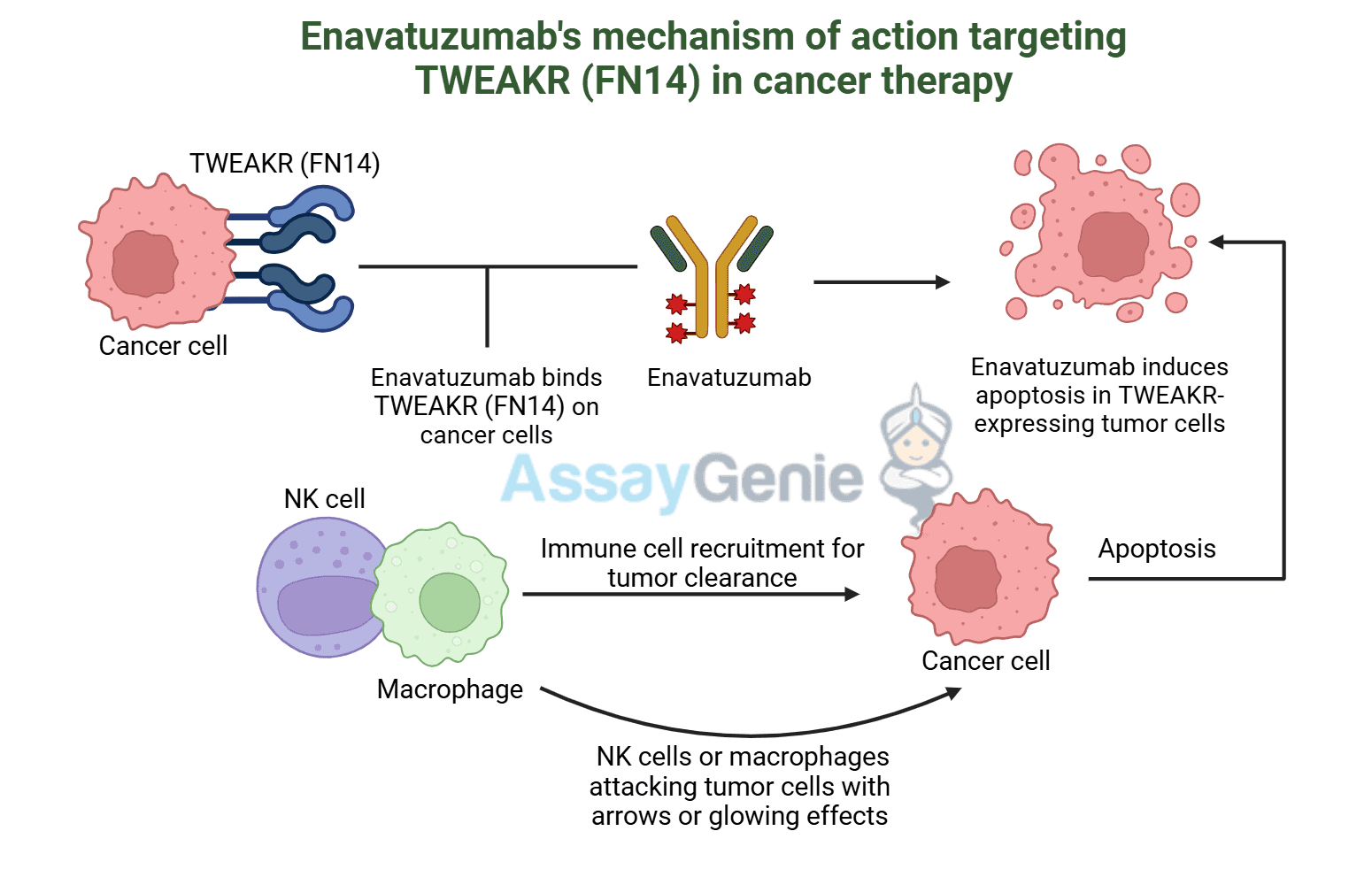
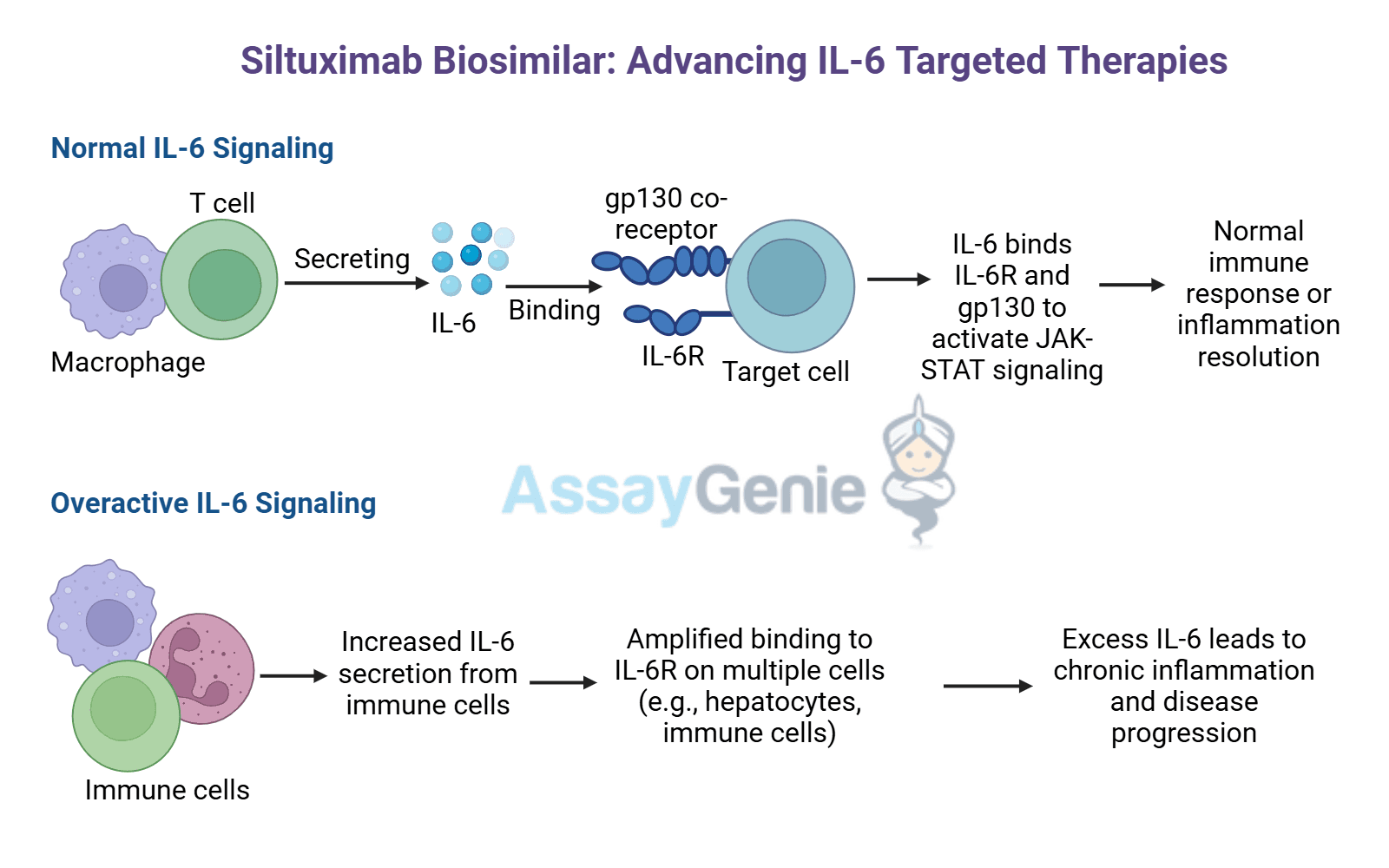
VivoGenie Biosimilar Antibodies
VivoGenie Biosimilars
Functional-Grade biosimilars for animal pre-clinical In Vivo studies

Why choose VivoGenie Biosimilar Antibodies?
Popular VivoGenie Biosimilars
Testimonials & Partners
Citations
Product | Author | Citation |
|---|---|---|
Bikoriman et al. | ||
Leane et al. | ||
Rohani et al. | ||
Calvo et al. | ||
Turley et al. | ||
Wang et al. |
cGMP & ISO Certification


Quality is at the core of everything we do.
Manufactured in state-of-the-art facilities, our products meet the highest standards, including current Good Manufacturing Practicies) cGMP, ISO 9001:2015, and ISO 13485:2016 certifications. These ensure consistent quality, customer-focused innovation, and reliable performance in every batch.
VivoGenie Biosimilar Data
QC Data




Figure 1. Quality control for different lots of PD-1 [RMP1-14] antibody. All the in vivo antibodies undergo extensive quality control ensuring extreme lot-to-lot reproducibility. Endotoxin data for eight lots demonstrate that all lots were below the specification of ≤ 1 EU/mg as determined by the limulus amebocyte lysate (LAL) method (Fig. 1A). Analysis by size exclusion chromatography visualized by superimposing chromatograms for all nine lots, with overlapping retention times and minimal baseline noise (Fig. 1B). All lots show high purity as shown by non-reducing and reducing SDS-PAGE (Fig. 1C and 1D).
Meet Some of the Genie Team!
Frequently Asked Questions
- Preclinical drug screening
- Assay development and validation (e.g., ELISA, Western Blot, Flow Cytometry)
- Mechanism of action studies
- Comparative studies against reference biologics