Description
ULK1 ELISA Sampler Kit
The ULK1 ELISA Kit Pack is a powerful tool for analyzing ULK1 protein levels in human samples. ULK1 is a key regulator of autophagy, a process essential for maintaining cellular homeostasis and responding to various stressors. With its high sensitivity and specificity, this kit provides accurate and reliable results, making it perfect for a wide range of research applications.ULK1 is involved in multiple cellular processes, including cell growth, differentiation, and survival. Dysregulation of autophagy and ULK1 has been linked to various diseases, such as cancer, neurodegenerative disorders, and metabolic disorders.
Therefore, studying ULK1 levels can provide valuable insights into these conditions and aid in the development of potential treatments.Overall, the ULK1 ELISA Kit Pack is a valuable tool for researchers looking to study autophagy, ULK1 function, and their implications in human health and disease. With its easy-to-use format and reliable performance, this kit is a must-have for any lab conducting research in the field of cellular biology and disease mechanisms.
ULK1 ELISA Sampler Pack
ULK1 ELISA Sampler Pack from Assay Genie allows researchers to begin their investigation into ULK1 via analysing PRKAA1 / AMPK Alpha 1, Regulatory-associated protein of mTOR, ULK1, AMPK alpha-2 / PRKAA2 in a 48 assay format, thus allowing researchers to identify and validate analytes within the pathway.
| Pack Contents | Size | Reactivity | Sensitivity | Range | ELISA Type |
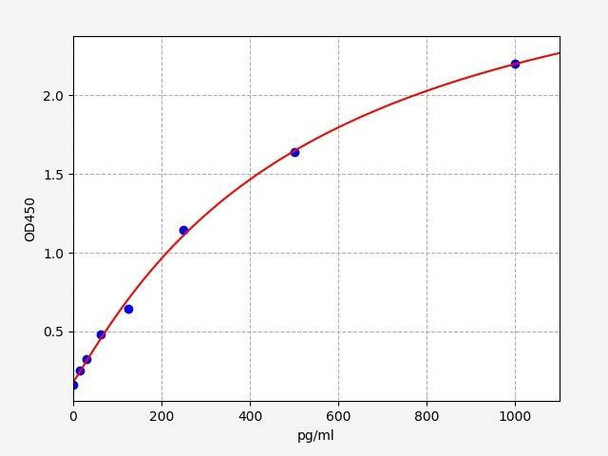
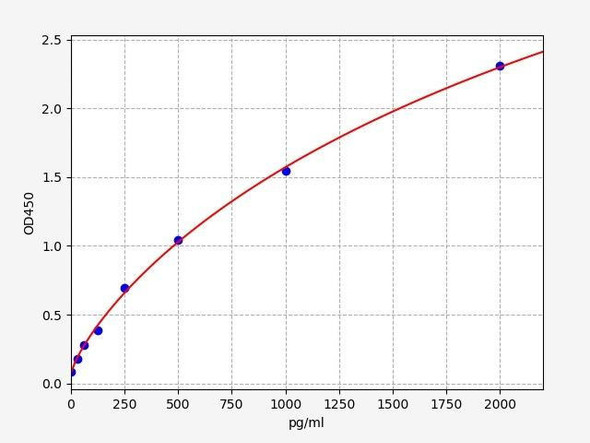
| Human PRKAA1 / AMPK Alpha 1 ELISA Kit | 48 Assays | Human | 46.875pg/ml | 78.125-5000pg/ml | Competitive ELISA |
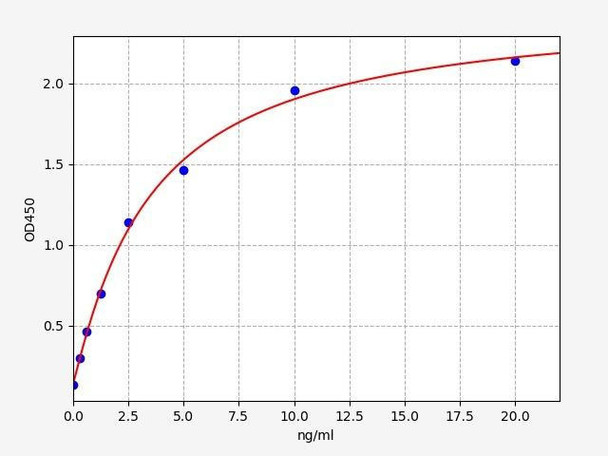
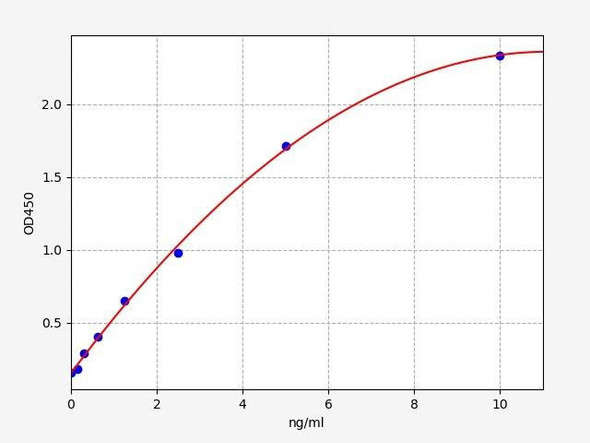
| Human Regulatory-associated protein of mTOR ELISA Kit | 48 Assays | Human | 0.188ng/ml | 0.313-20ng/ml | Sandwich ELISA |
| Human ULK1(Unc-51 Like Kinase 1) ELISA Kit | 48 Assays | Human | 9.375pg/ml | 15.625-1000pg/ml | Sandwich ELISA |
| Human AMPK alpha-2 / PRKAA2 ELISA Kit | 48 Assays | Human | 0.094ng/ml | 0.156-10ng/ml | Sandwich ELISA |
Each individual ELISA kit in this pack will come with the following components:
| Component | Quantity | Storage |
| ELISA Microplate (Dismountable) | 4-12 strips | 4°C for 6 months |
| Lyophilized Standard | 1 | 4°C/-20°C |
| Sample/Standard Dilution Buffer | 10ml | 4°C |
| Biotin-labeled Antibody(Concentrated) | 60ul | 4°C (Protect from light) |
| Antibody Dilution Buffer | 5ml | 4°C |
| HRP-Streptavidin Conjugate(SABC) | 60ul | 4°C (Protect from light) |
| SABC Dilution Buffer | 5ml | 4°C |
| TMB Substrate | 10ml | 4°C (Protect from light) |
| Stop Solution | 5ml | 4°C |
| Wash Buffer(25X) | 15ml | 4°C |
| Plate Sealer | 3 | - |
Other materials and equipment required:
- Microplate reader with 450 nm wavelength filter
- Multichannel Pipette, Pipette, microcentrifuge tubes and disposable pipette tips
- Incubator
- Deionized or distilled water
- Absorbent paper
- Buffer resevoir
When carrying out an ELISA assay it is important to prepare your samples in order to achieve the best possible results. Below we have a list of procedures for the preparation of samples for different sample types.
| Sample Type | Protocol |
| Serum | If using serum separator tubes, allow samples to clot for 30 minutes at room temperature. Centrifuge for 10 minutes at 1,000x g. Collect the serum fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. If serum separator tubes are not being used, allow samples to clot overnight at 2-8°C. Centrifuge for 10 minutes at 1,000x g. Remove serum and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. |
| Plasma | Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge samples at 4°C for 15 mins at 1000 - g within 30 mins of collection. Collect the plasma fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. Note: Over haemolysed samples are not suitable for use with this kit. |
| Urine & Cerebrospinal Fluid | Collect the urine (mid-stream) in a sterile container, centrifuge for 20 mins at 2000-3000 rpm. Remove supernatant and assay immediately. If any precipitation is detected, repeat the centrifugation step. A similar protocol can be used for cerebrospinal fluid. |
| Cell culture supernatant | Collect the cell culture media by pipette, followed by centrifugation at 4°C for 20 mins at 1500 rpm. Collect the clear supernatant and assay immediately. |
| Cell lysates | Solubilize cells in lysis buffer and allow to sit on ice for 30 minutes. Centrifuge tubes at 14,000 x g for 5 minutes to remove insoluble material. Aliquot the supernatant into a new tube and discard the remaining whole cell extract. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Tissue homogenates | The preparation of tissue homogenates will vary depending upon tissue type. Rinse tissue with 1X PBS to remove excess blood & homogenize in 20ml of 1X PBS (including protease inhibitors) and store overnight at ≤ -20°C. Two freeze-thaw cycles are required to break the cell membranes. To further disrupt the cell membranes you can sonicate the samples. Centrifuge homogenates for 5 mins at 5000xg. Remove the supernatant and assay immediately or aliquot and store at -20°C or -80°C. |
| Tissue lysates | Rinse tissue with PBS, cut into 1-2 mm pieces, and homogenize with a tissue homogenizer in PBS. Add an equal volume of RIPA buffer containing protease inhibitors and lyse tissues at room temperature for 30 minutes with gentle agitation. Centrifuge to remove debris. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Breast Milk | Collect milk samples and centrifuge at 10,000 x g for 60 min at 4°C. Aliquot the supernatant and assay. For long term use, store samples at -80°C. Minimize freeze/thaw cycles. |
*Note: Protocols are specific to each batch/lot. For the correct instructions please follow the protocol included in your kit.
Equilibrate the TMB substrate for at least 30 min at 37°C beforeuse. When diluting samples and reagents, they must be mixed completely andevenly. It is recommended to plot a standard curve for each test.
| Step | Protocol |
| 1. | Set standard, test sample and control (zero) wells on the pre-coatedplate respectively, and then, record their positions. It isrecommended to measure each standard and sample in duplicate. Washplate 2 times before adding standard, sample and control (zero) wells! |
| 2. | Add Sample and Biotin-detection antibody: Add 50µL of Standard, Blank or Sample per well. The blankwell is added with Sample Dilution Buffer. Immediately add 50 µL of biotin-labelled antibody workingsolution to each well. Cover with the plate sealer provided. Gently tap the plate to ensure thoroughmixing. Incubate for 45 minutes at 37°C. (Solutions are added to the bottom of micro-ELISA platewell, avoid touching plate walls and foaming). |
| 3. | Wash: Aspirate each well and wash, repeating the process three timesWash by filling each well with Wash Buffer (approximately 350µL)using a squirt bottle, multi-channel pipette, manifold dispenser orautomated washer. Complete removal of liquid at each step is essentialto good performance. After the last wash, remove any remaining WashBuffer by aspirating or decanting. Invert the plate and pat it againstthick clean absorbent paper. |
| 4. | HRP-Streptavidin Conjugate(SABC): Add 100µL of SABC workingsolution to each well. Cover with a new Plate sealer. Incubate for30minutes at 37°C. |
| 5. | Wash: Repeat the aspiration/wash process for five times. |
| 6. | TMB Substrate: Add 90µL of TMB Substrate to each well. Coverwith a new Plate sealer. Incubate for about 10-20 minutes at 37°C.Protect from light. The reaction time can be shortened or extendedaccording to the actual color change, but not more than 30minutes.When apparent gradient appeared in standard wells, you can terminatethe reaction. |
| 7. | Stop: Add 50µL of Stop Solution to each well. Color turn toyellow immediately. The adding order of stop solution should be as thesame as the substrate solution. |
| 8. | OD Measurement: Determine the optical density (OD Value) of each wellat once, using a microplate reader set to 450 nm. You should open themicroplate reader ahead, preheat the instrument, and set the testing parameters. |
*Note: Protocols are specific to each batch/lot. For the correct instructions please follow the protocol included in your kit.
Before adding to wells, equilibrate the SABC working solution and TMB substrate for at least 30 min at 37°C. When diluting samples and reagents, they must be mixed completely and evenly. It is recommended to plot a standard curve for each test.
| Step | Protocol |
| 1. | Set standard, test sample and control (zero) wells on the pre-coated plate respectively, and then, record their positions. It is recommended to measure each standard and sample in duplicate. Wash plate 2 times before adding standard, sample and control (zero) wells! |
| 2. | Aliquot 0.1ml standard solutions into the standard wells. |
| 3. | Add 0.1 ml of Sample / Standard dilution buffer into the control (zero) well. |
| 4. | Add 0.1 ml of properly diluted sample ( Human serum, plasma, tissue homogenates and other biological fluids.) into test sample wells. |
| 5. | Seal the plate with a cover and incubate at 37 °C for 90 min. |
| 6. | Remove the cover and discard the plate content, clap the plate on the absorbent filter papers or other absorbent material. Do NOT let the wells completely dry at any time. Wash plate X2. |
| 7. | Add 0.1 ml of Biotin- detection antibody working solution into the above wells (standard, test sample & zero wells). Add the solution at the bottom of each well without touching the side wall. |
| 8. | Seal the plate with a cover and incubate at 37°C for 60 min. |
| 9. | Remove the cover, and wash plate 3 times with Wash buffer. Let wash buffer rest in wells for 1 min between each wash. |
| 10. | Add 0.1 ml of SABC working solution into each well, cover the plate and incubate at 37°C for 30 min. |
| 11. | Remove the cover and wash plate 5 times with Wash buffer, and each time let the wash buffer stay in the wells for 1-2 min. |
| 12. | Add 90 µl of TMB substrate into each well, cover the plate and incubate at 37°C in dark within 10-20 min. (Note: This incubation time is for reference use only, the optimal time should be determined by end user.) And the shades of blue can be seen in the first 3-4 wells (with most concentrated standard solutions), the other wells show no obvious color. |
| 13. | Add 50 µl of Stop solution into each well and mix thoroughly. The color changes into yellow immediately. |
| 14. | Read the O.D. absorbance at 450 nm in a microplate reader immediately after adding the stop solution. |