Description
| Product Name: | SARS-CoV-2 NSP9 Recombinant Protein |
| Product Code: | RPES7052 |
| Size: | 50µg |
| Species: | SARS-CoV-2 |
| Expression Host: | E.coli |
| Synonyms: | SARS-CoV 2 nsp9,Single-stranded RNA-binding protein |
| Mol Mass: | 14.67kDa |
| AP Mol Mass: | 13-14 kDa |
| Tag: | N-His |
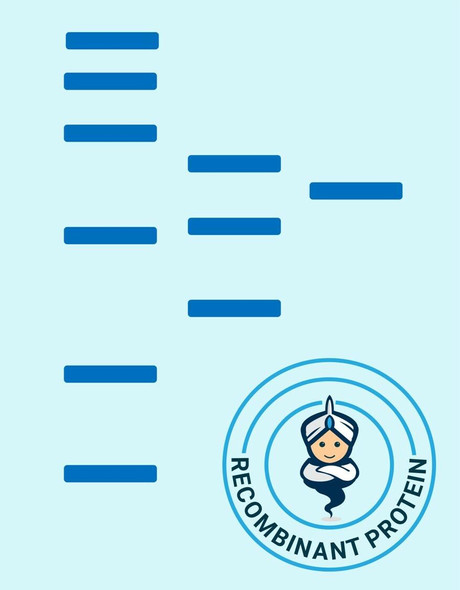
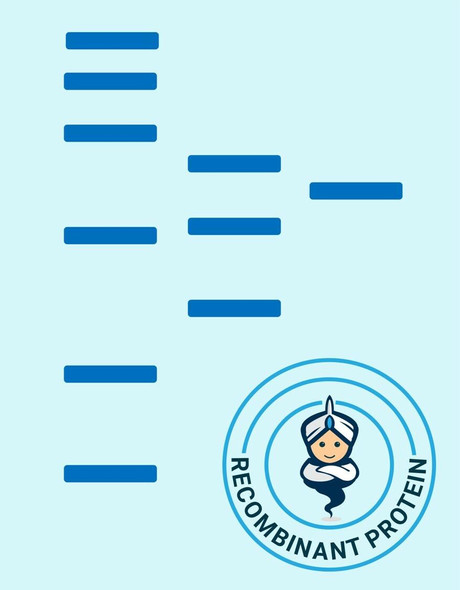
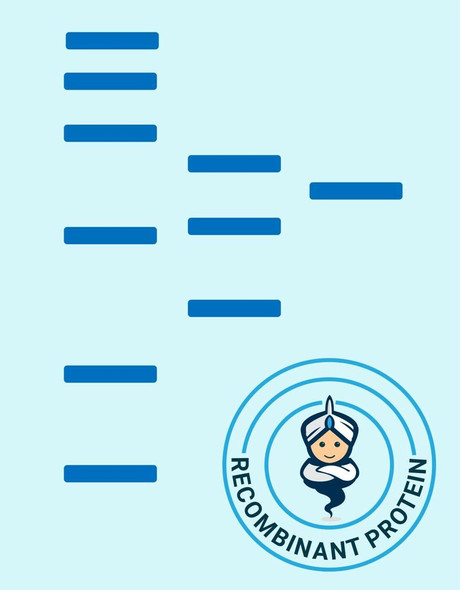
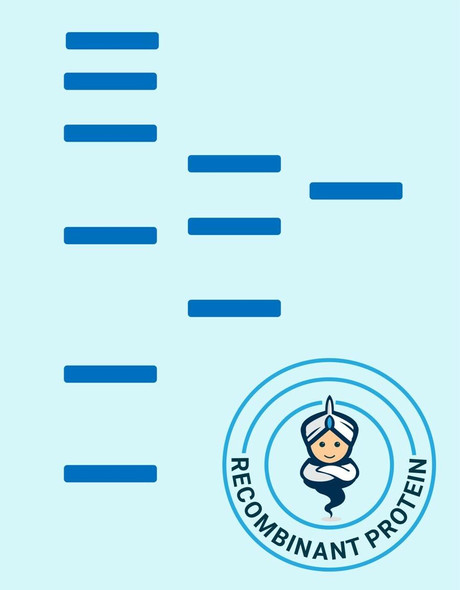
| Purity: | > 90 % as determined by reducing SDS-PAGE. |
| Endotoxin Level: | Please contact us for more information. |
| Bio Activity: | Testing in progress |
| Sequence: | Asn4141-Gln4253 |
| Accession: | QHD43415.1 |
| Storage: | Generally, lyophilized proteins are stable for up to 12 months when stored at -20 to -80°C. Reconstituted protein solution can be stored at 4-8°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Shipping: | This product is provided as lyophilized powder which is shipped with ice packs. |
| Formulation: | Supplied as solution form in PBS, pH7.5 or lyophilized from PBS, pH7.5 Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Please refer to the specific buffer information in the printed manual. |
| Reconstitution: | Please refer to the printed manual for detailed information. |
| Background: | During infection of human cells, SARS-CoV Non-structural protein 9 (Nsp9SARS) was found to be essential for replication. Homologs of the Nsp9 protein have been identified in numerous coronaviruses, including SARS-Cov-2 (Nsp9COV19), human coronavirus 229E (Nsp9HcoV), avian infectious bronchitis virus (Nsp9IBV), porcine epidemic diarrhea virus (Nsp9PEDV), and porcine delta virus (Nsp9PDCoV). Nsp9SARS has been shown to have modest affinity for long oligonucleotides with binding thought to be dependent on oligomerization state. Nsp9SARS dimerizes in solution via a conserved α-helical “GxxxG” motif. Disruption of key residues within this motif reduces both RNA binding and SARS-CoV viral replication. The mechanism of RNA binding within the Nsp9 protein family is not understood as these proteins have an unusual structural fold not previously seen in RNA-binding proteins. The fold's Greek-key motif exhibits topological similarities with Oligonucleotide/oligosaccharide binding proteins (OB-fold), but such vestiges have proven insufficient to provide clear insight into Nsp9 function. As a consequence of the weak affinity of Nsp9SARS for long oligonucleotide stretches it was suggested that the natural RNA substrate may instead be conserved features at the 3′ end of the viral-genome (the stem-loop II RNA-motif). Furthermore, potential direct interactions with the co-factors of the RNA polymerase have been reported. However, it remains to be determined how the oligonucleotide-binding activity of Nsp9 proteins promotes viral replication during infection. |