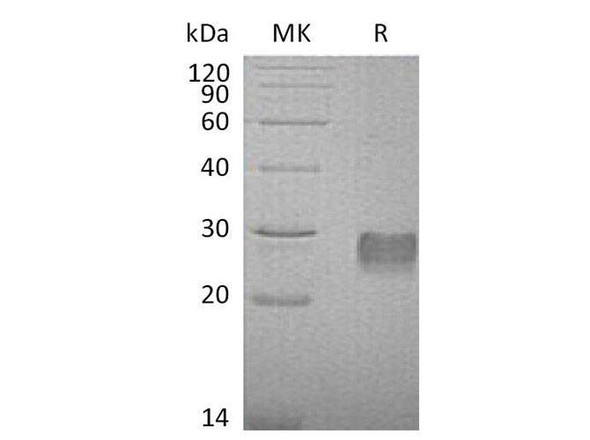
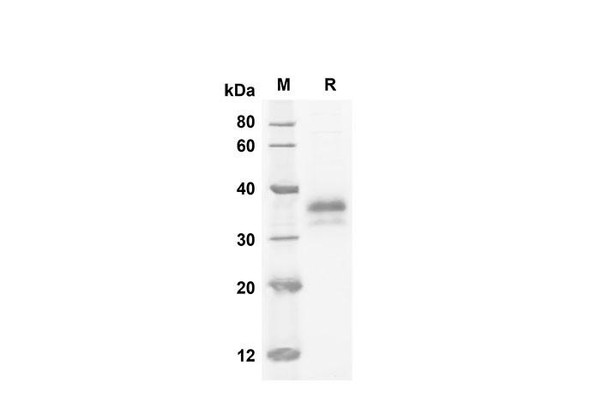
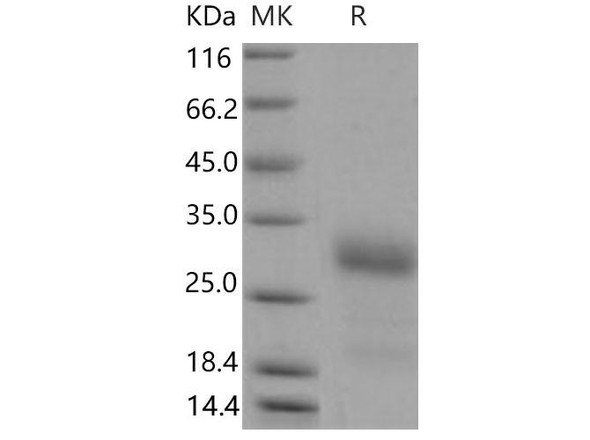
The superfamily of insulin-like growth factor (IGF) binding proteins include the six high-affinity IGF binding proteins (IGFBP) and at least four additional low-affinity binding proteins referred to as IGFBP related proteins (IGFBP-rP). All IGFBP superfamily members are cysteine-rich proteins with an conserved cysteine residues, which are clustered in the amino-and carboxy-terminal thirds of the molecule. IGFBPs modulate the biological activities of IGF proteins. Some IGFBPs may also have intrinsic bioactivity that is independent of their ability to bind IGF proteins. Post-translational modifications of IGFBPs, including glycosylation, phosphorylation and proteolysis, have been shown to modify the affinities of the binding proteins to IGF. Mouse IGFBP-6 cDNA encodes a 238 amino acid (aa) residue precursor protein with an a putative 25 aa residue signal peptide that is processed to generate the 213 aa residue mature protein that is O-glycosylated. Mouse and Human IGFBP-6 share 73% amino acid similarity. Mouse and rat IGFBP-6 share 94% amino acid similarity and the Mouse IGFBP-6 has a 9 amino acid insertion compared to the rat homolog. IGFBP-6 is expressed in ovarian, testicular, muscle, heart and lung tissues in the adult Mouse. IGFBP-6 was not detected in total RNA from a whole Mouse embryo.