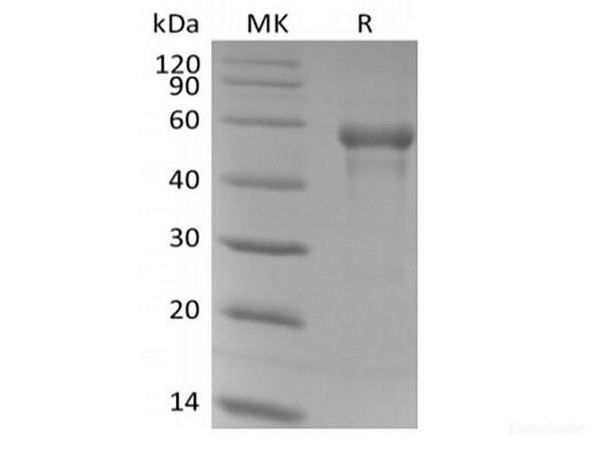
| Sequence: | His25-Ile334 |
| Accession: | Q9P2W7 |
| Storage: | Generally, lyophilized proteins are stable for up to 12 months when stored at -20 to -80°C. Reconstituted protein solution can be stored at 4-8°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Shipping: | This product is provided as lyophilized powder which is shipped with ice packs. |
| Formulation: | Lyophilized from a 0.2 μm filtered solution of 20mM Tris-HCl, 150mM NaCl, pH 8.0. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Please refer to the specific buffer information in the print |
| Reconstitution: | Please refer to the printed manual for detailed information. |
| Background: | B3GAT1 is the key enzyme during the biosynthesis of the carbohydrate epitope HNK-1, which is present on a number of cell adhesion molecules important in neurodevelopment. It adds a glucuronic residue to the terminal lactosamine residue (Gal beta 14GlcNAc) of a glycoprotein or glycolipid, which can be further sulfated to become the HNK1 epitope, a unique trisaccharide structure, HSO3-3GlcA beta 1-3Gal beta 1-4GlcNAc. The enzyme activity was found to be enhanced in the presence of sphingomyelin and phosphatidylinositol. The HNK1 carbohydrate epitope is characteristically expressed on a series of cell adhesion molecules in addition to some glycolipids in the extracellular matrix and on the cell surface in the nervous system, where it is involved in cell-cell and cell-substratum interaction and recognition during the development of the nervous system. Like most known glycosyltransferases, B3GAT1 is a type II Golgi-resident transmembrane protein with a short N-terminal cytoplasmic domain and a single pass transmembrane domain followed by an enzymatic domain in the lumen of Golgi apparatus. The enzyme activity was assayed using a phosphatase-coupled method. |