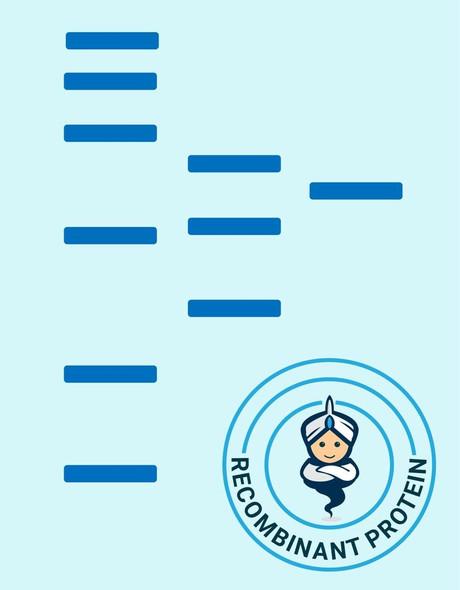
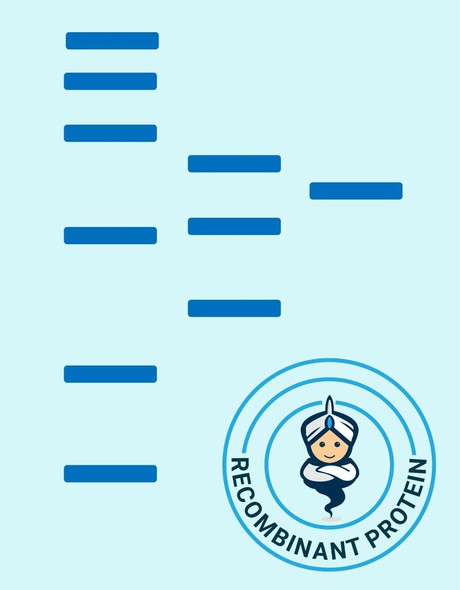
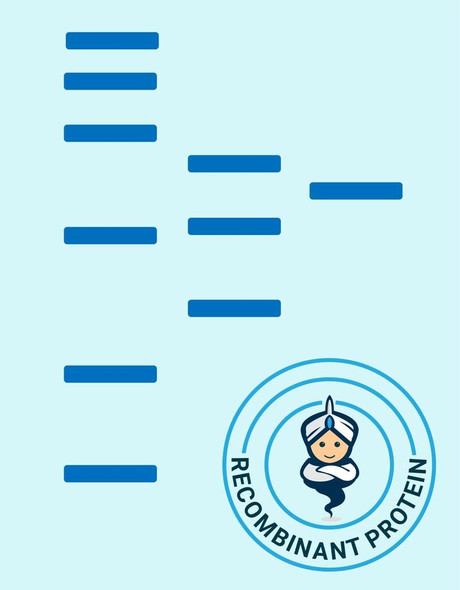
| Background: | EGF is the founding member of the EGF-family of proteins. Members of this protein family have highly similar structural and functional characteristics. EGF contains 9 EGF-like domains and 9 LDL-receptor class B repeats. Human EGF is a 645-Da protein with 53 amino acid residues and three intramolecular disulfide bonds. As a low-molecular-weight polypeptide, EGF was first purified from the mouse submandibular gland, but since then it was found in many human tissues including submandibular gland, parotid gland. It can also be found in human platelets, macrophages, urine, saliva, milk, and plasma. EGF is a growth factor that stimulates the growth of various epidermal and epithelial tissues in vivo and in vitro and of some fibroblasts in cell culture. It results in cellular proliferation, differentiation, and survival. Salivary EGF, which seems also regulated by dietary inorganic iodine, also plays an important physiological role in the maintenance of oro-esophageal and gastric tissue integrity. EGF acts by binding with high affinity to epidermal growth factor receptor on the cell surface and stimulating the intrinsic protein-tyrosine kinase activity of the receptor. The tyrosine kinase activity, in turn, initiates a signal transduction cascade that results in a variety of biochemical changes within the cell - a rise in intracellular calcium levels, increased glycolysis and protein synthesis, and increases in the expression of certain genes including the gene for EGFR - that ultimately lead to DNA synthesis and cell proliferation. |