Description
Background
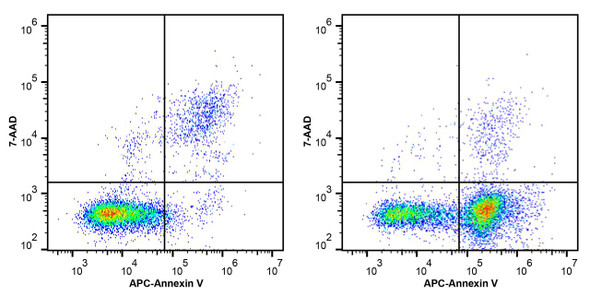
PI Reagent (50µg/mL) is developed to identify apoptotic and necrotic cells. Propidium Iodide (PI) is a common DNA dye that is not permeable to cell membrane. Once binding to DNA, the flurescence of PI increases by nearly 20 fold. Due to the loss of integrity of membrane, PI can enter late apoptotic or necrotic cells to stain DNA. Cells at different apoptotic stages can be distinguished by using Propidium Iodide (PI) and Annexin V.
- Induce apoptosis of suspension cells with reagents of interest. Collect cell cultures, centrifuge at 300 g for 5 min and discard the supernatant. Add PBS to wsh the cells and resuspend the cells gently followed by the cell counting.
- Split the cell suspension into tubes, 1-5 x 105 cells for each, centrifuge at 300 g for 5 min and discard the supernatant. Add PBS to wash the cells and discard the supernatant. Add 500 µL of 1 x Annexin V Binding Buffer to resuspend the cells.
- Add 5 µl of Annexin V-FITC and 5 µl of PI Reagent (50µg/mL) to each tube.
- Gently vortex the cells and incubate at room temperature for 15-20 min in the dark.
- Analyze the cells immediately with proper machine settings. Otherwise, place the cells on ice in the dark and analyze within 1 hour.
*Note: This product is only validated in suspension cells. Good cell viability is the key to the experiment. When the adherent cells are used for apoptotic detection, treatments like digestion may increase the ratio of necrotic or apoptotic cells and cause uncontrollable effects on the experimental results. Please be aware!
Storage
Store at 2-8°C for one year in dark.
Cautions
- For maximal assay performance, this reagent should be used within 12 months. Avoid freeze / thaw cycles.
- Detect apoptosis as soon as possible after staining to avoid the increase number of apoptosis or necrosis
- Avoid extended exposure of the samples to direct light to protect the fluorophores from quenching.
- For your safety and health, please wear the lab coat and disposable gloves before the experiments.