FKBP1A is a 12kDa protein initially discovered on an immune cells on the basis of its capability to bind as well as mediate the intracellular effect of the immunosuppressant FK506. FKBP1A is also known to mediate the action of Rapamycin-immunosuppressive agent.�FKBP1A is part of the family of immunophilins, which have in common high affinity for immunosuppressant drugs and a peptidyl-prolyl cis-trans isomerase (PPIase).�Activity which participates in folding of proline-containing protein.�In the absence of immunosuppressive ligands, FKBP1A is involved in intracellular calcium regulation by associating with 3 types of Ca2+ release channel complexes: skeletal ryanodine receptors, cardiac ryanodine receptors and the inositol 1,4,5-triphosphate receptor. FKBP1A also interact with TGF-beta type I receptor exerting an inhibitory effect on the TGF-beta signaling pathway. FKBP12 plays a role in modulation of ryanodine receptor isoform-1 (ryr-1), a component of the calcium release channel of skeletal muscle sarcoplasmic reticulum. FKBP1A increase the folding of proteins and catalyzes the cis-trans isomerization of proline imidic peptide bonds in oligopeptides.
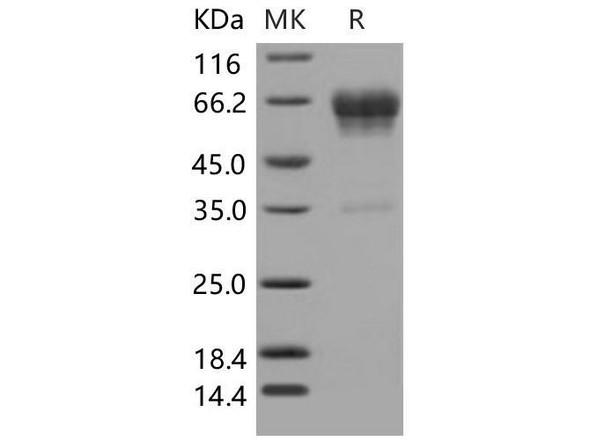
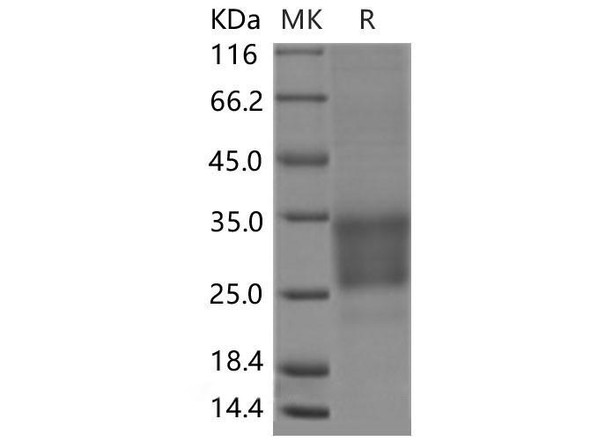
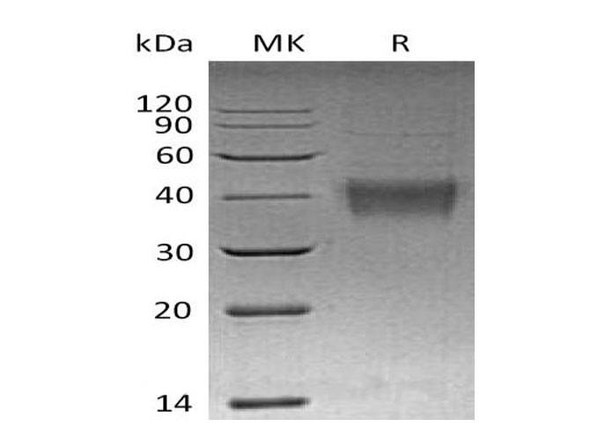
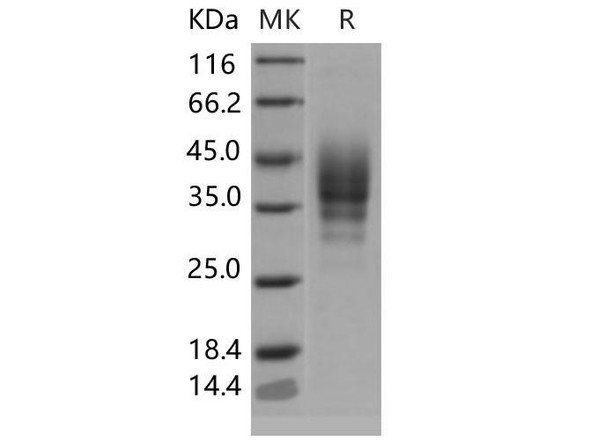
FKBP1A Mouse Recombinant produced in E.Coli is a single, non-glycosylated polypeptide chain containing 132 amino acids (1-108 a.a) and having a molecular mass of 14.4kDa.FKBP1A is fused to a 24 amino acid His-tag at N-terminus & purified by proprietary chromatographic techniques.