Description
Mouse Fascin (Fscn1) ELISA Kit
The Mouse Fascin (FSCN1) ELISA Kit is specifically designed to quantify levels of fascin protein in mouse serum, plasma, and cell culture supernatants with high accuracy. Fascin is a key protein involved in regulating actin filament bundling and is crucial for cell migration, adhesion, and invasion processes. This ELISA kit offers high sensitivity and specificity, ensuring reliable and reproducible results for researchers studying cell motility, cancer metastasis, and other cellular processes where fascin is implicated. By accurately measuring fascin levels, researchers can gain valuable insights into the role of this protein in various biological processes and diseases.
With its easy-to-use protocol and robust performance, the Mouse Fascin (FSCN1) ELISA Kit is an essential tool for elucidating the molecular mechanisms underlying cell movement and invasion, as well as for identifying potential therapeutic targets for diseases such as cancer. Order your kit today and accelerate your research efforts in the field of cell biology and cancer biology.
| Product Name: | Mouse Fascin (Fscn1) ELISA Kit |
| SKU: | MOEB1667 |
| Size: | 96T |
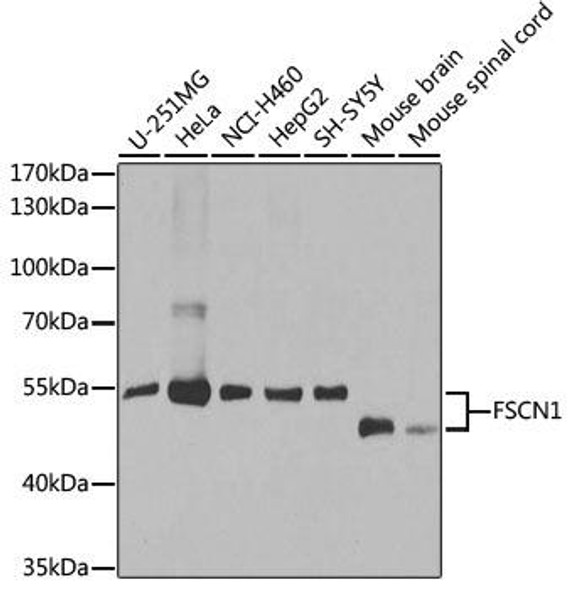
| Target: | Mouse Fascin (Fscn1) |
| Synonyms: | Singed-like protein, Fan1, Snl |
| Assay Type: | Sandwich |
| Detection Method: | ELISA |
| Reactivity: | Mouse |
| Detection Range: | 0.625-40ng/mL |
| Sensitivity: | 0.33ng/mL |
| Intra CV: | Provided with the Kit |
| Inter CV: | Provided with the Kit |
| Linearity: | Provided with the Kit |
| Recovery: | Provided with the Kit |
| Function: | Organizes filamentous actin into bundles with a minimum of 4.1:1 actin/fascin ratio. Plays a role in the organization of actin filament bundles and the formation of microspikes, membrane ruffles, and stress fibers. Important for the formation of a diverse set of cell protrusions, such as filopodia, and for cell motility and migration. |
| Uniprot: | Q61553 |
| Sample Type: | Serum, plasma, tissue homogenates, cell culture supernates and other biological fluids |
| Specificity: | Natural and recombinant mouse Fascin |
| Sub Unit: | Interacts with RUFY3 (via N-terminus); the interaction induces neuron axon development (PubMed:24720729). Associates with beta-catenin (PubMed:8794867). Interacts with PLXNB3 (PubMed:21706053). |
| Research Area: | Epigenetics |
| Subcellular Location: | Cytoplasm Cytosol Cytoplasm Cytoskeleton Cell projection Growth cone Cell projection Filopodium Cell projection Invadopodium Cell projection Microvillus Cell junction Colocalized with RUFY3 and F-actin at filipodia of the axonal growth cone (PubMed:24720729). Colocalized with DBN1 and F-actin at the transitional domain of the axonal growth cone (PubMed:24720729). In glioma cells, partially colocalizes with F-actin stress fibers in the cytosol (By similarity). |
| Storage: | Please see kit components below for exact storage details |
| Note: | For research use only |
| UniProt Protein Function: | fascin: Organizes filamentous actin into bundles with a minimum of 4.1:1 actin/fascin ratio. Plays a role in the organization of actin filament bundles and the formation of microspikes, membrane ruffles, and stress fibers. Important for the formation of a diverse set of cell protrusions, such as filopodia, and for cell motility and migration. Associates with beta-catenin. Ubiquitous. Belongs to the fascin family. |
| UniProt Protein Details: | Protein type:Actin-binding; Cytoskeletal; Motility/polarity/chemotaxis Chromosomal Location of Human Ortholog: 5 G2|5 81.84 cM Cellular Component: actin cytoskeleton; cell junction; cell projection; cell projection membrane; cytoplasm; cytoskeleton; cytosol; dendrite; extracellular exosome; filamentous actin; filopodium; growth cone; intercellular junction; invadopodium; lamellipodium; microspike; microvillus; myelin sheath; plasma membrane; podosome; ruffle; stress fiber Molecular Function:actin binding; actin filament binding; cadherin binding; drug binding; protein binding; protein binding, bridging; RNA binding Biological Process: actin cytoskeleton organization; actin filament bundle formation; actin filament organization; anatomical structure morphogenesis; cell migration; cell motility; establishment and/or maintenance of cell polarity; establishment of apical/basal cell polarity; intercellular junction assembly; microspike assembly; positive regulation of extracellular matrix disassembly; positive regulation of filopodium formation; positive regulation of lamellipodium assembly; positive regulation of podosome assembly; regulation of actin cytoskeleton organization; regulation of microvillus assembly |
| UniProt Code: | Q61553 |
| NCBI GenInfo Identifier: | 113680348 |
| NCBI Gene ID: | 14086 |
| NCBI Accession: | NP_032010.2 |
| UniProt Secondary Accession: | Q61553,O09099, O09156, Q05DK3, Q7TN32, Q80V75, |
| UniProt Related Accession: | Q61553 |
| Molecular Weight: | 54,508 Da |
| NCBI Full Name: | fascin |
| NCBI Synonym Full Names: | fascin actin-bundling protein 1 |
| NCBI Official Symbol: | Fscn1 |
| NCBI Official Synonym Symbols: | Fan1; AI663989; fascin-1 |
| NCBI Protein Information: | fascin |
| UniProt Protein Name: | Fascin |
| UniProt Synonym Protein Names: | Singed-like protein |
| UniProt Gene Name: | Fscn1 |
| Component | Quantity (96 Assays) | Storage |
| ELISA Microplate (Dismountable) | 8×12 strips | -20°C |
| Lyophilized Standard | 2 | -20°C |
| Sample Diluent | 20ml | -20°C |
| Assay Diluent A | 10mL | -20°C |
| Assay Diluent B | 10mL | -20°C |
| Detection Reagent A | 120µL | -20°C |
| Detection Reagent B | 120µL | -20°C |
| Wash Buffer | 30mL | 4°C |
| Substrate | 10mL | 4°C |
| Stop Solution | 10mL | 4°C |
| Plate Sealer | 5 | - |
Other materials and equipment required:
- Microplate reader with 450 nm wavelength filter
- Multichannel Pipette, Pipette, microcentrifuge tubes and disposable pipette tips
- Incubator
- Deionized or distilled water
- Absorbent paper
- Buffer resevoir
*Note: The below protocol is a sample protocol. Protocols are specific to each batch/lot. For the correct instructions please follow the protocol included in your kit.
Allow all reagents to reach room temperature (Please do not dissolve the reagents at 37°C directly). All the reagents should be mixed thoroughly by gently swirling before pipetting. Avoid foaming. Keep appropriate numbers of strips for 1 experiment and remove extra strips from microtiter plate. Removed strips should be resealed and stored at -20°C until the kits expiry date. Prepare all reagents, working standards and samples as directed in the previous sections. Please predict the concentration before assaying. If values for these are not within the range of the standard curve, users must determine the optimal sample dilutions for their experiments. We recommend running all samples in duplicate.
| Step | |
| 1. | Add Sample: Add 100µL of Standard, Blank, or Sample per well. The blank well is added with Sample diluent. Solutions are added to the bottom of micro ELISA plate well, avoid inside wall touching and foaming as possible. Mix it gently. Cover the plate with sealer we provided. Incubate for 120 minutes at 37°C. |
| 2. | Remove the liquid from each well, don't wash. Add 100µL of Detection Reagent A working solution to each well. Cover with the Plate sealer. Gently tap the plate to ensure thorough mixing. Incubate for 1 hour at 37°C. Note: if Detection Reagent A appears cloudy warm to room temperature until solution is uniform. |
| 3. | Aspirate each well and wash, repeating the process three times. Wash by filling each well with Wash Buffer (approximately 400µL) (a squirt bottle, multi-channel pipette,manifold dispenser or automated washer are needed). Complete removal of liquid at each step is essential. After the last wash, completely remove remaining Wash Buffer by aspirating or decanting. Invert the plate and pat it against thick clean absorbent paper. |
| 4. | Add 100µL of Detection Reagent B working solution to each well. Cover with the Plate sealer. Incubate for 60 minutes at 37°C. |
| 5. | Repeat the wash process for five times as conducted in step 3. |
| 6. | Add 90µL of Substrate Solution to each well. Cover with a new Plate sealer and incubate for 10-20 minutes at 37°C. Protect the plate from light. The reaction time can be shortened or extended according to the actual color change, but this should not exceed more than 30 minutes. When apparent gradient appears in standard wells, user should terminatethe reaction. |
| 7. | Add 50µL of Stop Solution to each well. If color change does not appear uniform, gently tap the plate to ensure thorough mixing. |
| 8. | Determine the optical density (OD value) of each well at once, using a micro-plate reader set to 450 nm. User should open the micro-plate reader in advance, preheat the instrument, and set the testing parameters. |
| 9. | After experiment, store all reagents according to the specified storage temperature respectively until their expiry. |
When carrying out an ELISA assay it is important to prepare your samples in order to achieve the best possible results. Below we have a list of procedures for the preparation of samples for different sample types.
| Sample Type | Protocol |
| Serum | If using serum separator tubes, allow samples to clot for 30 minutes at room temperature. Centrifuge for 10 minutes at 1,000x g. Collect the serum fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. If serum separator tubes are not being used, allow samples to clot overnight at 2-8°C. Centrifuge for 10 minutes at 1,000x g. Remove serum and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. |
| Plasma | Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge samples at 4°C for 15 mins at 1000 × g within 30 mins of collection. Collect the plasma fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. Note: Over haemolysed samples are not suitable for use with this kit. |
| Urine & Cerebrospinal Fluid | Collect the urine (mid-stream) in a sterile container, centrifuge for 20 mins at 2000-3000 rpm. Remove supernatant and assay immediately. If any precipitation is detected, repeat the centrifugation step. A similar protocol can be used for cerebrospinal fluid. |
| Cell culture supernatant | Collect the cell culture media by pipette, followed by centrifugation at 4°C for 20 mins at 1500 rpm. Collect the clear supernatant and assay immediately. |
| Cell lysates | Solubilize cells in lysis buffer and allow to sit on ice for 30 minutes. Centrifuge tubes at 14,000 x g for 5 minutes to remove insoluble material. Aliquot the supernatant into a new tube and discard the remaining whole cell extract. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Tissue homogenates | The preparation of tissue homogenates will vary depending upon tissue type. Rinse tissue with 1X PBS to remove excess blood & homogenize in 20ml of 1X PBS (including protease inhibitors) and store overnight at ≤ -20°C. Two freeze-thaw cycles are required to break the cell membranes. To further disrupt the cell membranes you can sonicate the samples. Centrifuge homogenates for 5 mins at 5000xg. Remove the supernatant and assay immediately or aliquot and store at -20°C or -80°C. |
| Tissue lysates | Rinse tissue with PBS, cut into 1-2 mm pieces, and homogenize with a tissue homogenizer in PBS. Add an equal volume of RIPA buffer containing protease inhibitors and lyse tissues at room temperature for 30 minutes with gentle agitation. Centrifuge to remove debris. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Breast Milk | Collect milk samples and centrifuge at 10,000 x g for 60 min at 4°C. Aliquot the supernatant and assay. For long term use, store samples at -80°C. Minimize freeze/thaw cycles. |