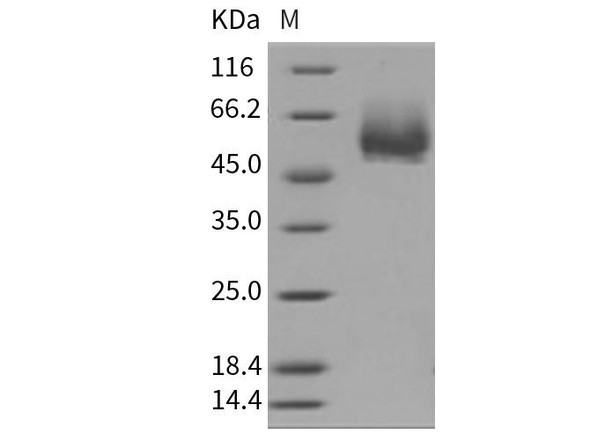
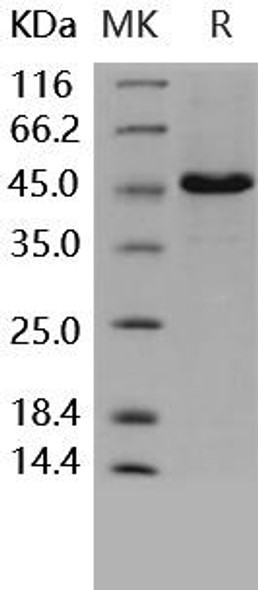
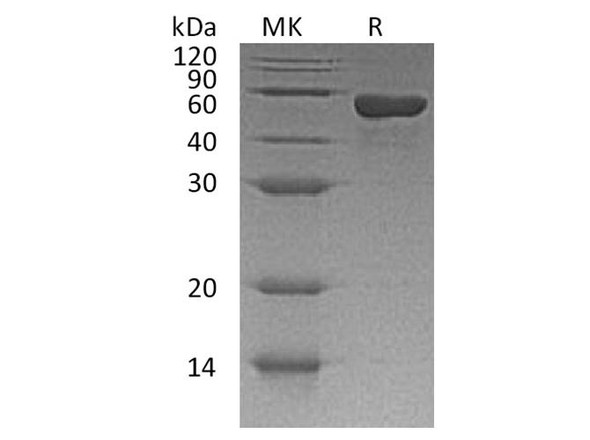
| Background: | T-cell surface glycoprotein CD4, is a single-pass type I membrane protein. CD4 contains three Ig-like C2-type (immunoglobulin-like) domains and one Ig-like V-type (immunoglobulin-like) domain. CD4 is a glycoprotein expressed on the surface of T helper cells, regulatory T cells, monocytes, macrophages, and dendritic cells. The CD4 surface determinant, previously associated as a phenotypic marker for helper/inducer subsets of T lymphocytes, has now been critically identified as the binding/entry protein for human immunodeficiency viruses (HIV). The human CD4 molecule is readily detectable on monocytes, T lymphocytes, and brain tissues. All human tissue sources of CD4 bind radiolabeled gp120 to the same relative degree; however, the murine homologous protein, L3T4, does not bind the HIV envelope protein. CD4 is a co-receptor that assists the T cell receptor (TCR) to activate its T cell following an interaction with an antigen-presenting cell. Using its portion that resides inside the T cell, CD4 amplifies the signal generated by the TCR. CD4 interacts directly with MHC class II molecules on the surface of the antigen-presenting cell via its extracellular domain. The CD4 molecule is currently the object of intense interest and investigation both because of its role in normal T-cell function, and because of its role in HIV infection. CD4 is a primary receptor used by HIV-1 to gain entry into host T cells. HIV infection leads to a progressive reduction of the number of T cells possessing CD4 receptors. |