Mouse BMP4 ELISA Kit (MOFI00127)
- SKU:
- MOFI00127
- Product Type:
- ELISA Kit
- Size:
- 96 Assays
- Uniprot:
- P21275
- Sensitivity:
- 18.75pg/ml
- Range:
- 31.25-2000pg/ml
- ELISA Type:
- Sandwich
- Synonyms:
- BMP-4, Bone Morphogenetic Protein 4, BMP2B, BMP2B1, MCOPS6, ZYME, DVR4, OFC11, ZYME
- Reactivity:
- Mouse
- Research Area:
- Cell Biology
Description
Mouse BMP4 ELISA Kit
The Mouse BMP4 (Bone Morphogenetic Protein 4) ELISA Kit is specially designed for the accurate measurement of BMP4 levels in mouse serum, plasma, and tissue homogenates. This kit offers high sensitivity and specificity, ensuring precise and consistent results for various research purposes.BMP4 is a key regulator of cell differentiation and growth, playing a vital role in embryonic development, tissue regeneration, and bone formation.
Dysregulation of BMP4 has been implicated in various diseases, including skeletal disorders, cancer, and inflammatory conditions, making it a valuable biomarker for disease research and therapeutic development.With its reliable performance and user-friendly protocols, the Mouse BMP4 ELISA Kit is an indispensable tool for scientists studying BMP4 signaling pathways and exploring its potential applications in biomedical research.
| Product Name: | Mouse BMP4 ELISA Kit |
| Product Code: | MOFI00127 |
| Size: | 96 Assays |
| Alias: | BMP-4, Bone Morphogenetic Protein 4, BMP2B, BMP2B1, MCOPS6, ZYME, DVR4, OFC11, ZYME |
| Detection Method: | Sandwich ELISA |
| Application: | This immunoassay kit allows for the in vitro quantitative determination of Mouse BMP-4 concentrations in serum plasma and other biological fluids. |
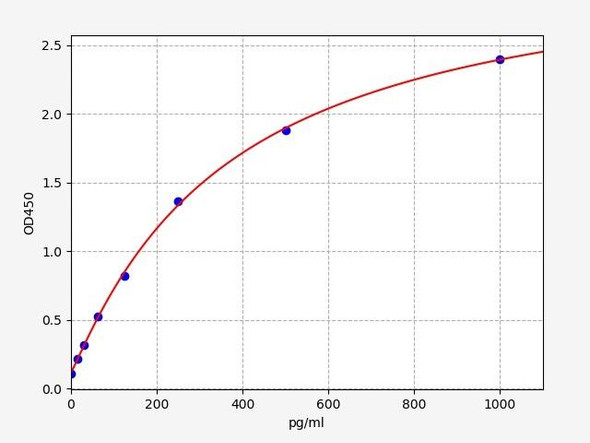
| Sensitivity: | 18.75pg/ml |
| Range: | 31.25-2000pg/ml |
| Storage: | 4°C for 6 months |
| Note: | For Research Use Only |
| Recovery: | Matrices listed below were spiked with certain level of Mouse BMP-4 and the recovery rates were calculated by comparing the measured value to the expected amount of Mouse BMP-4 in samples. | ||||||||||||||||
| |||||||||||||||||
| Linearity: | The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Mouse BMP-4 and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected. | ||||||||||||||||
| |||||||||||||||||
| Intra Assay: | CV <8% | ||||||||||||||||
| Inter Assay: | CV <10% |
| Component | Quantity | Storage |
| ELISA Microplate (Dismountable) | 8×12 strips | 4°C for 6 months |
| Lyophilized Standard | 2 | 4°C/-20°C |
| Sample/Standard Dilution Buffer | 20ml | 4°C |
| Biotin-labeled Antibody(Concentrated) | 120ul | 4°C (Protect from light) |
| Antibody Dilution Buffer | 10ml | 4°C |
| HRP-Streptavidin Conjugate(SABC) | 120ul | 4°C (Protect from light) |
| SABC Dilution Buffer | 10ml | 4°C |
| TMB Substrate | 10ml | 4°C (Protect from light) |
| Stop Solution | 10ml | 4°C |
| Wash Buffer(25X) | 30ml | 4°C |
| Plate Sealer | 5 | - |
Other materials and equipment required:
- Microplate reader with 450 nm wavelength filter
- Multichannel Pipette, Pipette, microcentrifuge tubes and disposable pipette tips
- Incubator
- Deionized or distilled water
- Absorbent paper
- Buffer resevoir
| Uniprot | P21275 |
| UniProt Protein Function: | BMP4: Induces cartilage and bone formation. Also act in mesoderm induction, tooth development, limb formation and fracture repair. Acts in concert with PTHLH/PTHRP to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Homodimer; disulfide-linked. Interacts with GREM2. Part of a complex consisting of TWSG1 and CHRD. Interacts with the serine proteases, HTRA1 and HTRA3; the interaction with either inhibits BMP4-mediated signaling. The HTRA protease activity is required for this inhibition. Interacts with SOSTDC1. Expressed in the lung and lower levels seen in the kidney. Present also in normal and neoplastic prostate tissues, and prostate cancer cell lines. Belongs to the TGF-beta family. |
| UniProt Protein Details: | Protein type:Secreted, signal peptide; Secreted Cellular Component: extracellular space; proteinaceous extracellular matrix; cytoplasm; extracellular region Molecular Function:heparin binding; protein binding; protein homodimerization activity; growth factor activity; cytokine activity; transforming growth factor beta receptor binding; chemoattractant activity Biological Process: negative regulation of MAP kinase activity; activation of MAPKK activity; positive regulation of apoptosis; embryonic skeletal development; positive regulation of transcription, DNA-dependent; negative regulation of chondrocyte differentiation; mesodermal cell differentiation; telencephalon regionalization; cardiac muscle cell differentiation; germ cell development; BMP signaling pathway; regulation of protein import into nucleus; anatomical structure formation; kidney development; regulation of odontogenesis of dentine-containing teeth; endochondral ossification; embryonic limb morphogenesis; positive regulation of cardiac muscle fiber development; negative regulation of immature T cell proliferation in the thymus; cell fate commitment; camera-type eye development; regulation of smooth muscle cell proliferation; neuron fate commitment; camera-type eye morphogenesis; regulation of gene expression; negative regulation of mitosis; positive regulation of epidermal cell differentiation; smooth muscle cell differentiation; positive regulation of transcription from RNA polymerase II promoter; embryonic digit morphogenesis; negative regulation of apoptosis; tissue development; positive regulation of protein binding; negative regulation of transcription from RNA polymerase II promoter; negative regulation of cell proliferation; inner ear receptor cell differentiation; ureteric bud development; intermediate mesodermal cell differentiation; forebrain development; positive regulation of cell proliferation; angiogenesis; embryonic morphogenesis; positive regulation of BMP signaling pathway; common-partner SMAD protein phosphorylation; negative regulation of T cell differentiation in the thymus; positive regulation of bone mineralization; positive regulation of ossification; odontogenesis of dentine-containing teeth; embryonic skeletal morphogenesis; osteoblast differentiation; positive regulation of osteoblast differentiation; blood vessel endothelial cell proliferation during sprouting angiogenesis; telencephalon development; ureteric bud branching; regulation of cell fate commitment; positive regulation of neuron differentiation; lung development; anterior/posterior axis specification; renal system process; macrophage differentiation; multicellular organismal development; heart development; lymphoid progenitor cell differentiation; post-embryonic development; positive regulation of endothelial cell differentiation; positive chemotaxis; induction of an organ; chondrocyte differentiation; erythrocyte differentiation; specification of organ position; regulation of endothelial cell proliferation; monocyte differentiation; embryonic cranial skeleton morphogenesis; negative regulation of striated muscle development; mesoderm formation; branching morphogenesis of a tube; negative regulation of phosphorylation; steroid hormone mediated signaling; hemopoietic progenitor cell differentiation; positive regulation of endothelial cell proliferation; negative regulation of transcription, DNA-dependent; positive regulation of cell differentiation; metanephros development; alveolus development; positive regulation of epithelial cell proliferation; positive regulation of smooth muscle cell proliferation; positive regulation of collagen biosynthetic process; embryonic hindlimb morphogenesis; positive regulation of vascular endothelial growth factor receptor signaling pathway; negative regulation of cell cycle; odontogenesis; smooth muscle development; ovarian follicle development; vasculature development; regulation of cell differentiation; regulation of smooth muscle cell differentiation; cell differentiation; skeletal development; dorsoventral neural tube patterning; negative regulation of epithelial cell proliferation; blood vessel development; ossification; negative regulation of oligodendrocyte differentiation; eye development; pituitary gland development; cartilage development; neural tube closure; positive regulation of protein amino acid phosphorylation; negative regulation of myoblast differentiation; mesodermal cell fate determination; growth |
| UniProt Code: | P21275 |
| NCBI GenInfo Identifier: | 461633 |
| NCBI Gene ID: | 12159 |
| NCBI Accession: | P21275.2 |
| UniProt Related Accession: | P21275 |
| Molecular Weight: | |
| NCBI Full Name: | Bone morphogenetic protein 4 |
| NCBI Synonym Full Names: | bone morphogenetic protein 4 |
| NCBI Official Symbol: | Bmp4 |
| NCBI Official Synonym Symbols: | Bmp-4; Bmp2b; Bmp2b1; Bmp2b-1 |
| NCBI Protein Information: | bone morphogenetic protein 4; bone morphogenetic protein 2B |
| UniProt Protein Name: | Bone morphogenetic protein 4 |
| UniProt Synonym Protein Names: | Bone morphogenetic protein 2B; BMP-2B |
| UniProt Gene Name: | Bmp4 |
| UniProt Entry Name: | BMP4_MOUSE |
*Note: Protocols are specific to each batch/lot. For the correct instructions please follow the protocol included in your kit.
| Step | Procedure |
| 1. | Set standard, test sample and control (zero) wells on the pre-coated plate respectively, and then, record their positions. It is recommended to measure each standard and sample in duplicate. Wash plate 2 times before adding standard, sample and control (zero) wells! |
| 2. | Aliquot 0.1ml standard solutions into the standard wells. |
| 3. | Add 0.1 ml of Sample / Standard dilution buffer into the control (zero) well. |
| 4. | Add 0.1 ml of properly diluted sample (Human serum, plasma, tissue homogenates and other biological fluids.) into test sample wells. |
| 5. | Seal the plate with a cover and incubate at 37 °C for 90 min. |
| 6. | Remove the cover and discard the plate content, clap the plate on the absorbent filter papers or other absorbent material. Do NOT let the wells completely dry at any time. Wash plate X2. |
| 7. | Add 0.1 ml of Biotin- detection antibody working solution into the above wells (standard, test sample & zero wells). Add the solution at the bottom of each well without touching the side wall. |
| 8. | Seal the plate with a cover and incubate at 37°C for 60 min. |
| 9. | Remove the cover, and wash plate 3 times with Wash buffer. Let wash buffer rest in wells for 1 min between each wash. |
| 10. | Add 0.1 ml of SABC working solution into each well, cover the plate and incubate at 37°C for 30 min. |
| 11. | Remove the cover and wash plate 5 times with Wash buffer, and each time let the wash buffer stay in the wells for 1-2 min. |
| 12. | Add 90 µL of TMB substrate into each well, cover the plate and incubate at 37°C in dark within 10-20 min. (Note: This incubation time is for reference use only, the optimal time should be determined by end user.) And the shades of blue can be seen in the first 3-4 wells (with most concentrated standard solutions), the other wells show no obvious color. |
| 13. | Add 50 µL of Stop solution into each well and mix thoroughly. The color changes into yellow immediately. |
| 14. | Read the O.D. absorbance at 450 nm in a microplate reader immediately after adding the stop solution. |
When carrying out an ELISA assay it is important to prepare your samples in order to achieve the best possible results. Below we have a list of procedures for the preparation of samples for different sample types.
| Sample Type | Protocol |
| Serum: | If using serum separator tubes, allow samples to clot for 30 minutes at room temperature. Centrifuge for 10 minutes at 1,000x g. Collect the serum fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. If serum separator tubes are not being used, allow samples to clot overnight at 2-8°C. Centrifuge for 10 minutes at 1,000x g. Remove serum and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. |
| Plasma: | Collect plasma using EDTA or heparin as an anticoagulant. Centrifuge samples at 4°C for 15 mins at 1000 × g within 30 mins of collection. Collect the plasma fraction and assay promptly or aliquot and store the samples at -80°C. Avoid multiple freeze-thaw cycles. Note: Over haemolysed samples are not suitable for use with this kit. |
| Urine & Cerebrospinal Fluid: | Collect the urine (mid-stream) in a sterile container, centrifuge for 20 mins at 2000-3000 rpm. Remove supernatant and assay immediately. If any precipitation is detected, repeat the centrifugation step. A similar protocol can be used for cerebrospinal fluid. |
| Cell culture supernatant: | Collect the cell culture media by pipette, followed by centrifugation at 4°C for 20 mins at 1500 rpm. Collect the clear supernatant and assay immediately. |
| Cell lysates: | Solubilize cells in lysis buffer and allow to sit on ice for 30 minutes. Centrifuge tubes at 14,000 x g for 5 minutes to remove insoluble material. Aliquot the supernatant into a new tube and discard the remaining whole cell extract. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20°C. |
| Tissue homogenates: | The preparation of tissue homogenates will vary depending upon tissue type. Rinse tissue with 1X PBS to remove excess blood & homogenize in 20ml of 1X PBS (including protease inhibitors) and store overnight at ≤ -20°C. Two freeze-thaw cycles are required to break the cell membranes. To further disrupt the cell membranes you can sonicate the samples. Centrifuge homogenates for 5 mins at 5000xg. Remove the supernatant and assay immediately or aliquot and store at -20°C or -80°C. |
| Tissue lysates: | Rinse tissue with PBS, cut into 1-2 mm pieces, and homogenize with a tissue homogenizer in PBS. Add an equal volume of RIPA buffer containing protease inhibitors and lyse tissues at room temperature for 30 minutes with gentle agitation. Centrifuge to remove debris. Quantify total protein concentration using a total protein assay. Assay immediately or aliquot and store at ≤ -20 °C. |
| Breast Milk: | Collect milk samples and centrifuge at 10,000 x g for 60 min at 4°C. Aliquot the supernatant and assay. For long term use, store samples at -80°C. Minimize freeze/thaw cycles. |