Ipilimumab and Nivolumab: Advancing Research on CTLA-4 in Cancer Therapy
What You Need to Know About Ipilimumab
What is Ipilimumab?
Ipilimumab is a monoclonal antibody that targets CTLA-4, a protein involved in downregulating the immune system, to boost the body's immune response against cancer cells.
How does Ipilimumab work?
By inhibiting CTLA-4, ipilimumab enhances T-cell activation and proliferation, enabling a stronger immune response to attack tumors.
What are the side effects of Ipilimumab?
Common side effects include fatigue, diarrhea, rash, and more severe immune-related adverse events like colitis and hepatitis.
What is the role of Ipilimumab in combination with Nivolumab?
The combination of ipilimumab and nivolumab has shown enhanced efficacy in treating advanced melanoma and other cancers by targeting two different immune checkpoints.
1.) Understanding Ipilimumab
Ipilimumab, marketed under the brand name Yervoy, is a pioneering immunotherapy drug approved for treating various cancers, including metastatic melanoma. It was the first immune checkpoint inhibitor to receive FDA approval and has become a cornerstone in immuno-oncology. Ipilimumab works by targeting CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), a receptor on T-cells that downregulates immune responses. By blocking CTLA-4, ipilimumab unleashes the immune system to attack cancer cells more effectively.
2.) Mechanism of Action of Ipilimumab
Ipilimumab’s mechanism centers on inhibiting CTLA-4, a critical checkpoint in T-cell regulation. CTLA-4 competes with CD28 for binding to B7 ligands on antigen-presenting cells. When CTLA-4 binds to B7, it suppresses T-cell activity, preventing an overactive immune response. Ipilimumab blocks this interaction, allowing CD28 to bind B7 and activate T-cells. This activation enables a robust immune attack on tumors, making ipilimumab effective in combating cancers like melanoma.
3.) Clinical Applications of Ipilimumab
Ipilimumab’s primary indication is for metastatic melanoma, but it has also shown efficacy in treating renal cell carcinoma, colorectal cancer, and non-small cell lung cancer. When combined with nivolumab, another immune checkpoint inhibitor targeting PD-1, ipilimumab has demonstrated improved survival rates in advanced melanoma and other cancers. Emerging studies continue to explore its potential in combination therapies and earlier-stage disease settings.
4.) Advancing Research on Ipilimumab
What is a Biosimilar?
Biosimilars are biologic medical products highly similar to an original reference product, with no clinically meaningful differences in terms of safety, purity, and potency. They offer a cost-effective alternative for research and therapeutic purposes while maintaining rigorous standards.
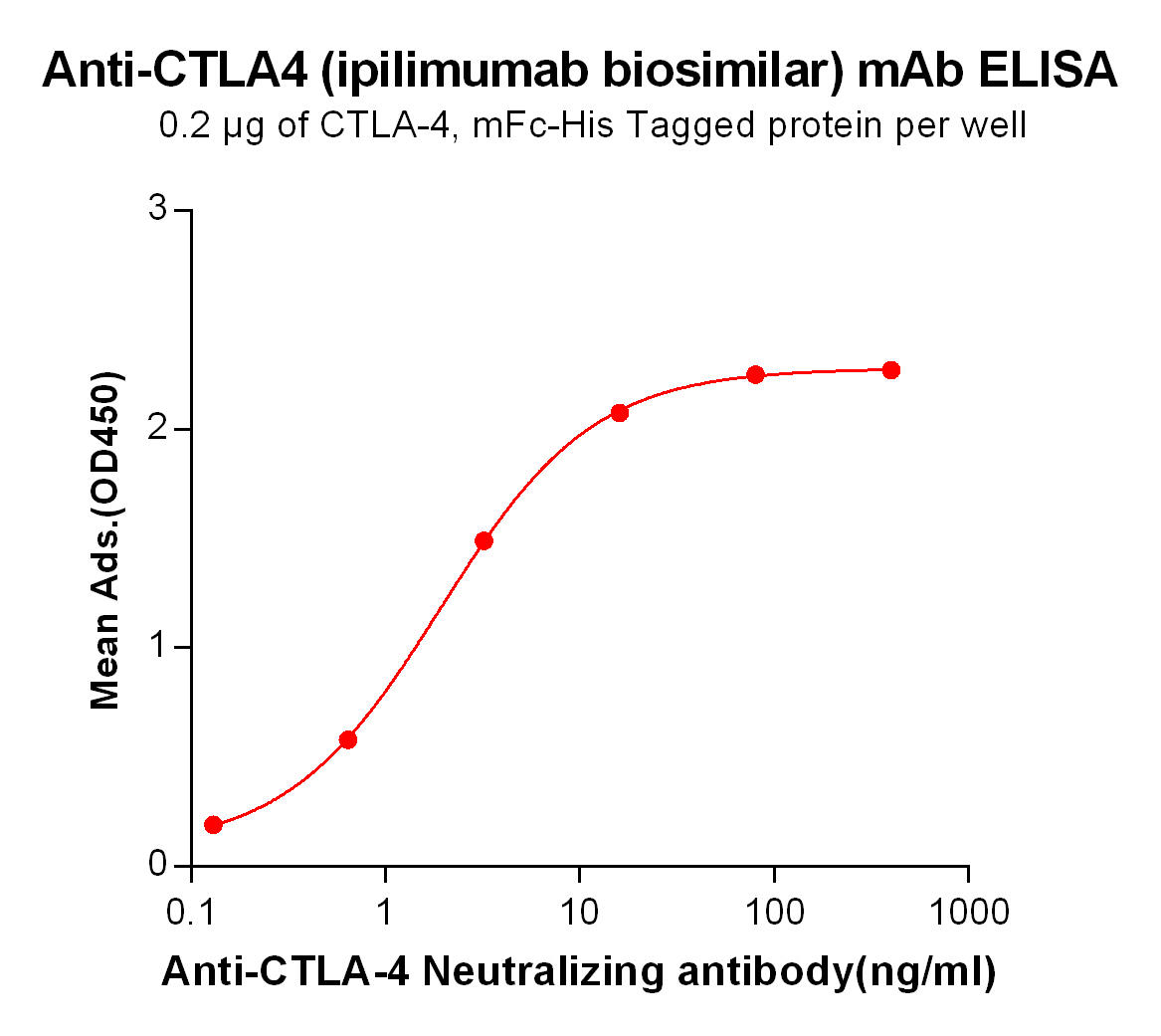
| Ipilimumab (Anti-CTLA-4) Biosimilar Antibody | |
|---|---|
| Antibody Type: | Monoclonal Antibody |
| Protein: | CTLA-4 |
| Reactivity: | Human |
How Our Ipilimumab Biosimilar Contributes to Research
Our Ipilimumab biosimilar is designed for research use only, providing scientists with a valuable tool to advance their studies. By mimicking the structure and function of the original drug, it enables exploration of CTLA-4 inhibition and its effects in various experimental models.
Benefits of Ipilimumab Biosimilar for Research
Cost-Effective Access: Enables extensive preclinical research without the high costs associated with branded drugs.
High Quality: Maintains rigorous standards to ensure reliability in research settings.
Versatile Applications: Useful for studying immune checkpoint pathways, drug interactions, and combination therapies.
Research Use Only Disclaimer
Our ipilimumab biosimilar is intended strictly for research purposes and is not approved for clinical or patient use. This distinction ensures its application is focused on advancing scientific knowledge and innovation.

Wrote by Zainab Riaz
Recent Posts
-
Tigatuzumab Biosimilar: Harnessing DR5 for Targeted Cancer Therapy
Tigatuzumab is a monoclonal antibody targeting death receptor 5 (DR5), a member of the …17th Dec 2025 -
Alemtuzumab Biosimilar: Advancing CD52-Targeted Therapy
Alemtuzumab is a monoclonal antibody targeting CD52, a glycoprotein highly expressed on …17th Dec 2025 -
Enavatuzumab Biosimilar: Advancing TWEAKR-Targeted Therapy in Cancer
Enavatuzumab is a monoclonal antibody targeting TWEAK receptor (TWEAKR, also known as F …17th Dec 2025




