Phospholipase A2 (PLA2) catalyzes the hydrolysis of the sn-2 position of membrane glycerophospholipids to liberate arachidonic acid (AA), a precursor of eicosanoids including prostaglandins and leukotrienes. The same reaction also produces lysophosholipids, which represent another class of lipid mediators.The secretory PLA2 (sPLA2) family, in which 10 isozymes have been identified, consists of low molecular weight, Ca2+-requiring secretory enzymes that have been implicated in a number of biological processes, such as modification of eicosanoid generation, inflammation, and host defense.This enzyme has been proposed to hydrolyze phosphatidylcholine (PC) in lipoproteins to liberate lyso-PC and free fatty acids in the arterial wall, thereby facilitating the accumulation of bioactive lipids and modified lipoproteins in atherosclerotic foci.In mice, sPLA2 expression significantly influences HDL particle size and composition and demonstrate that an induction of sPLA2 is required for the decrease in plasma HDL cholesterol in response to inflammatory stimuli. Instillation of bacteria into the bronchi was associated with surfactant degradation and a decrease in large:small ratio of surfactant aggregates in rats.
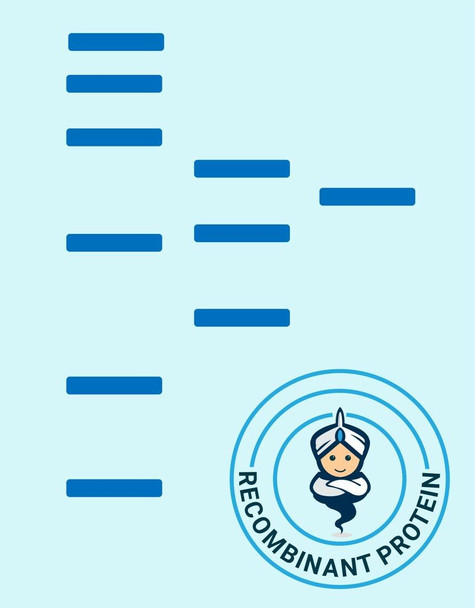
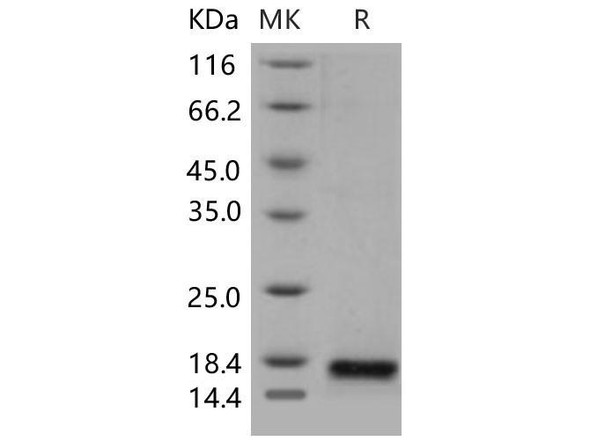
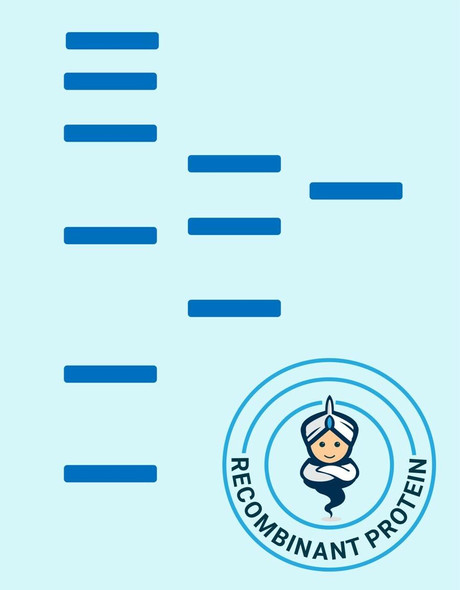
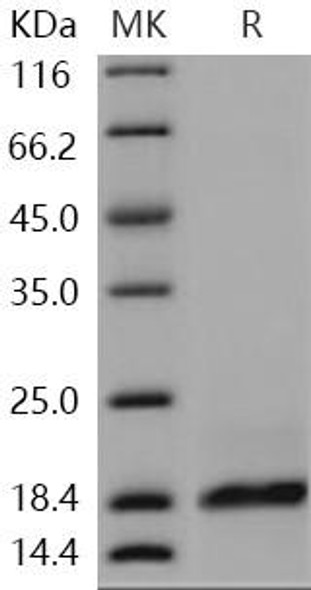
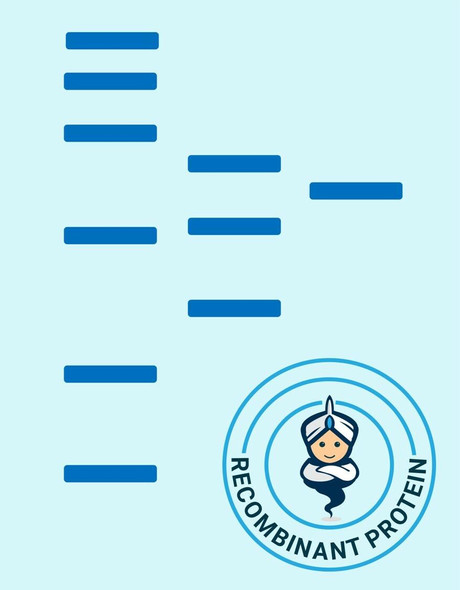
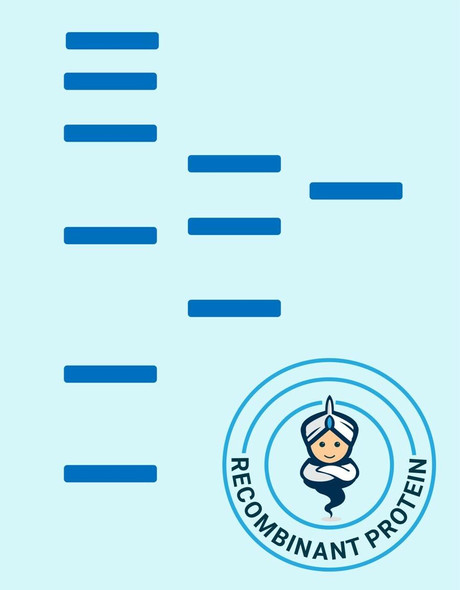
Secreted Phospholipase A2-IIE Human Recombinant manufactured with N-terminal His-Tag. PLA2G2E His-Tagged Fusion Protein is 15.8 kDa protein containing 123 amino acid residues of the human secreted phospholipase A2-IIE and 16 additional amino acid residues � His-Tag (underlined).