Description
| Product Name: | Human HMOX1 Recombinant Protein |
| Product Code: | RPPB1816 |
| Size: | 50µg |
| Species: | Human |
| Target: | HMOX1 |
| Synonyms: | HO-1, HSP32, bK286B10, HMOX-1, Heme oxygenase 1, HMOX1, HO, HO1. |
| Source: | Escherichia Coli |
| Physical Appearance: | Sterile filtered colorless solution. |
| Formulation: | HMOX1 1 mg/ml solution containing 20mM Tris-HCl pH-8, 50mM NaCl, 0.1mM PMSF and 10% glycerol. |
| Stability: | Store at 4°C if entire vial will be used within 2-4 weeks. Store, frozen at -20°C for longer periods of time. For long term storage it is recommended to add a carrier protein (0.1% HSA or BSA).Avoid multiple freeze-thaw cycles. |
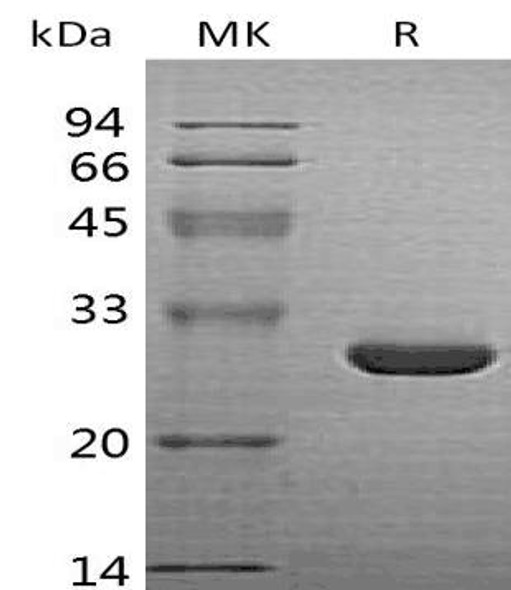
| Purity: | Greater than 95.0% as determined by SDS-PAGE. |
| Amino Acid Sequence: | MERPQPHSMP QDLSEALKEA TKEVHTQAEN AEFMRNFQKG QVTRDGFKLV MASLYHIYVA LEEEIERNKE SPVFAPVYFP EELHRKAALEQDLAFWYGPR WQEVIPYTPA MQRYVKRLHE VGRTEPELLV AHAYTRYLGD LSGGQVLKKI AQKALDLPSS GEGLAFFTFP NIASATKFKQLYRSRMNSLE MTPAVRQRVI EEAKTAFLLN IQLFEELQEL LTHDTKDQSP SRAPGLRQRA SNKVQDSAPV ETPRGKPPLN TRSQAPLEHHHHHH |
HMOX1 cleaves the heme ring at the alpha methene bridge to form Biliverdin. Biliverdin is then converted to Bilirubin by Biliverdin reductase. In physiological state, the highest activity of HMOX1 is found in the spleen, where senescent erythrocytes are sequestrated and destroyed. Heme Oxygenase-1 is involved in the regulation of cardiovascular function and its adaptive response to a variety of stressors. HMOX1 is induced in the colon of ulcerative colitis. HMOX1 is found to overexpress with a higher extent of intraplaque angiogenesis implies a multi-faceted role for HMOX1 in modulating the progression of atherosclerosis. HMOX1 expression reduced LPS-stimulated secretion of MCP-1, IL-6, IL-10, and TNF-alpha in murine and human macrophages.
HO-1 Human Recombinant protein produced in E.Coli is a single, non-glycosylated, polypeptide chain containing 274 amino acids (1-266) and having a molecular mass of 31.4 kDa. HO-1 is fused to an 8 amino acid His Tag at C-Terminus and purified by proprietary chromatographic techniques.
| UniProt Protein Function: | HMOX1: Heme oxygenase cleaves the heme ring at the alpha methene bridge to form biliverdin. Biliverdin is subsequently converted to bilirubin by biliverdin reductase. Under physiological conditions, the activity of heme oxygenase is highest in the spleen, where senescent erythrocytes are sequestrated and destroyed. Heme oxygenase 1 activity is highly inducible by its substrate heme and by various non-heme substances such as heavy metals, bromobenzene, endotoxin, oxidizing agents and UVA. Expressed at higher levels in renal cancer tissue than in normal tissue. Belongs to the heme oxygenase family. |
| UniProt Protein Details: | Protein type:EC 1.14.99.3; Oxidoreductase; Cofactor and Vitamin Metabolism - porphyrin and chlorophyll Chromosomal Location of Human Ortholog: 22q13.1 Cellular Component: caveola; cytosol; endoplasmic reticulum; endoplasmic reticulum membrane; extracellular space; membrane; nucleolus; nucleus; perinuclear region of cytoplasm Molecular Function:enzyme binding; heme binding; heme oxygenase (decyclizing) activity; metal ion binding; phospholipase D activity; protein binding; protein homodimerization activity; signal transducer activity Biological Process: angiogenesis; cell death; cellular iron ion homeostasis; cellular response to nutrient; DNA damage response, signal transduction resulting in induction of apoptosis; endothelial cell proliferation; erythrocyte homeostasis; excretion; healing during inflammatory response; heme catabolic process; heme oxidation; iron ion homeostasis; negative regulation of DNA binding; negative regulation of leukocyte migration; negative regulation of mast cell cytokine production; negative regulation of mast cell degranulation; negative regulation of neuron apoptosis; negative regulation of smooth muscle cell proliferation; negative regulation of transcription factor activity; porphyrin metabolic process; positive regulation of angiogenesis; positive regulation of chemokine biosynthetic process; positive regulation of I-kappaB kinase/NF-kappaB cascade; positive regulation of smooth muscle cell proliferation; positive regulation of vasodilation; protein homooligomerization; regulation of angiogenesis; regulation of blood pressure; regulation of transcription factor activity; regulation of transcription from RNA polymerase II promoter in response to oxidative stress; response to estrogen stimulus; response to hydrogen peroxide; response to nicotine; response to oxidative stress; small GTPase mediated signal transduction; smooth muscle hyperplasia; transmembrane transport Disease: Heme Oxygenase 1 Deficiency; Pulmonary Disease, Chronic Obstructive |
| NCBI Summary: | Heme oxygenase, an essential enzyme in heme catabolism, cleaves heme to form biliverdin, which is subsequently converted to bilirubin by biliverdin reductase, and carbon monoxide, a putative neurotransmitter. Heme oxygenase activity is induced by its substrate heme and by various nonheme substances. Heme oxygenase occurs as 2 isozymes, an inducible heme oxygenase-1 and a constitutive heme oxygenase-2. HMOX1 and HMOX2 belong to the heme oxygenase family. [provided by RefSeq, Jul 2008] |
| UniProt Code: | P09601 |
| NCBI GenInfo Identifier: | 123446 |
| NCBI Gene ID: | 3162 |
| NCBI Accession: | P09601.1 |
| UniProt Related Accession: | P09601 |
| Molecular Weight: | 32,819 Da |
| NCBI Full Name: | Heme oxygenase 1 |
| NCBI Synonym Full Names: | heme oxygenase 1 |
| NCBI Official Symbol: | HMOX1�� |
| NCBI Official Synonym Symbols: | HO-1; HSP32; HMOX1D; bK286B10�� |
| NCBI Protein Information: | heme oxygenase 1 |
| UniProt Protein Name: | Heme oxygenase 1 |
| UniProt Gene Name: | HMOX1�� |
| UniProt Entry Name: | HMOX1_HUMAN |