Description
| Product Name: | Human HEXA Recombinant Protein |
| Product Code: | RPPB1810 |
| Size: | 10µg |
| Species: | Human |
| Target: | HEXA |
| Synonyms: | HexosaminidaseA (Alpha Polypeptide), N-Acetyl-Beta-Glucosaminidase Subunit Alpha, Beta-N-AcetylhexosaminidaseSubunit Alpha, Hexosaminidase Subunit A, EC 3.2.1.52, TSD, Beta-HexosaminidaseSubunit Alpha, GM2 Gangliosidosis, Tay Sachs Disease, EC 3.2.1, Beta-hexosaminidasesubunit alpha, Beta-N-acetylhexosaminidase subunit alpha, Hexosaminidasesubunit A, N-acetyl-beta-glucosaminidase subunit alpha. |
| Source: | Sf9 Insect cells |
| Physical Appearance: | Sterile Filtered colorless solution. |
| Formulation: | HEXA protein solution (0. 5mg/ml) contains phosphatebuffered saline (pH7.4). |
| Stability: | Store at 4°C if entire vial will be used within 2-4 weeks. Store, frozen at -20°C for longer periods of time. For long term storage it is recommended to add a carrier protein (0.1% HSA or BSA).Avoid multiple freeze-thaw cycles. |
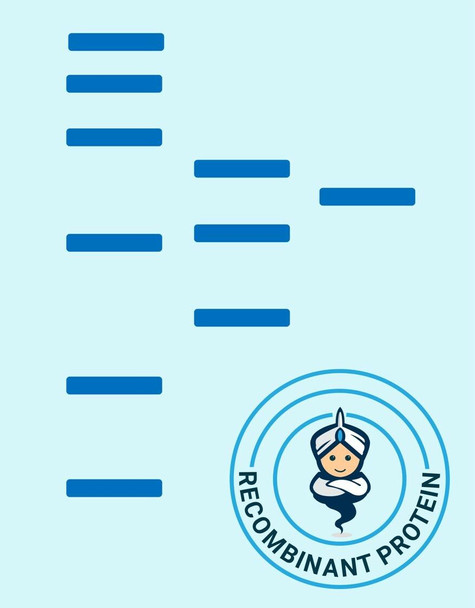
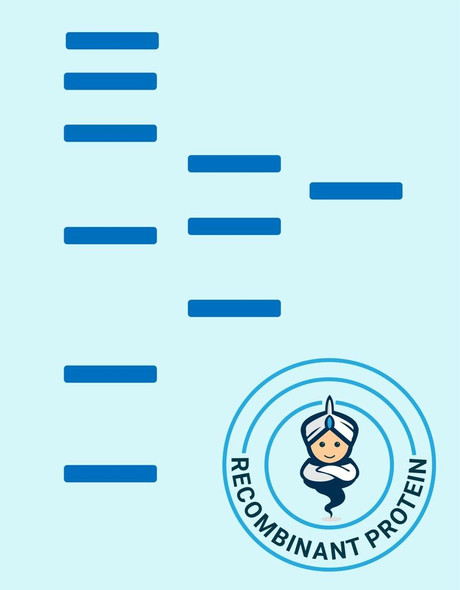
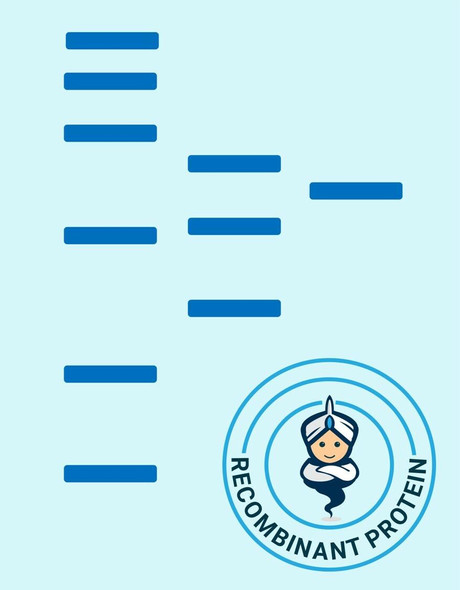
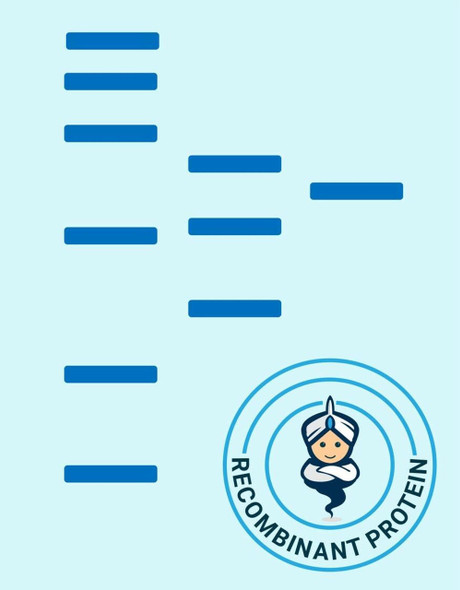
| Purity: | Greater than 90% as determined by SDS-PAGE. |
| Amino Acid Sequence: | LWPWPQNFQTSDQRYVLYPN NFQFQYDVSS AAQPGCSVLD EAFQRYRDLL FGSGSWPRPY LTGKRHTLEK NVLVVSVVTPGCNQLPTLES VENYTLTIND DQCLLLSETV WGALRGLETF SQLVWKSAEG TFFINKTEIE DFPRFPHRGLLLDTSRHYLP LSSILDTLDV MAYNKLNVFH WHLVDDPSFP YESFTFPELM RKGSYNPVTH IYTAQDVKEVIEYARLRGIR VLAEFDTPGH TLSWGPGIPG LLTPCYSGSE PSGTFGPVNP SLNNTYEFMS TFFLEVSSVFPDFYLHLGGD EVDFTCWKSN PEIQDFMRKK GFGEDFKQLE SFYIQTLLDI VSSYGKGYVV WQEVFDNKVKIQPDTIIQVW REDIPVNYMK ELELVTKAGF RALLSAPWYL NRISYGPDWK DFYIVEPLAF EGTPEQKALVIGGEACMWGE YVDNTNLVPR LWPRAGAVAE RLWSNKLTSD LTFAYERLSH FRCELLRRGV QAQPLNVGFCEQEFEQTHHH HHH |
HEXA is the alpha subunit of the lysosomal enzyme beta-hexosaminidase which, combined with the cofactor GM2 activator protein, catalyzes the degradation of the ganglioside GM2, and other molecules having N-acetyl hexosamines terminus. The two subunits composing Beta-hexosaminidase, alpha and beta, belong to the glycosyl hydrolases family and are encoded by distinct genes. Alpha subunit gene mutations can cause Tay-Sachs disease (GM2-gangliosidosis type I).
HEXA produced in Sf9 Baculovirus cells is a single,glycosylated polypeptide chain containing 513 amino acids (23-529a.a.) andhaving a molecular mass of 59.2kDa.�(Molecular size on SDS-PAGE will appear atapproximately 50-70kDa).�HEXA is expressed with a 6 amino acid His tag atC-Terminus and purified by proprietary chromatographic techniques.
| UniProt Protein Function: | HEXA: Responsible for the degradation of GM2 gangliosides, and a variety of other molecules containing terminal N-acetyl hexosamines, in the brain and other tissues. The form B is active against certain oligosaccharides. The form S has no measurable activity. Defects in HEXA are the cause of GM2-gangliosidosis type 1 (GM2G1); also known as Tay-Sachs disease. GM2- gangliosidosis is an autosomal recessive lysosomal storage disease marked by the accumulation of GM2 gangliosides in the neuronal cells. GM2G1 is characterized by GM2 gangliosides accumulation in the absence of HEXA activity, leading to neurodegeneration and, in the infantile form, death in early childhood. GM2G1 has an increased incidence among Ashkenazi Jews and French Canadians in eastern Quebec. It exists in several forms: infantile (most common and most severe), juvenile and adult (late onset). Belongs to the glycosyl hydrolase 20 family. |
| UniProt Protein Details: | Protein type:Glycan Metabolism - glycosphingolipid biosynthesis - ganglio series; Glycan Metabolism - glycosaminoglycan degradation; Carbohydrate Metabolism - amino sugar and nucleotide sugar; Hydrolase; Glycan Metabolism - other glycan degradation; Glycan Metabolism - glycosphingolipid biosynthesis - globo series; EC 3.2.1.52 Chromosomal Location of Human Ortholog: 15q24.1 Cellular Component: lysosomal lumen; membrane Molecular Function:protein heterodimerization activity; beta-N-acetylhexosaminidase activity Biological Process: keratan sulfate metabolic process; chondroitin sulfate metabolic process; sphingolipid metabolic process; glycosaminoglycan metabolic process; chondroitin sulfate catabolic process; carbohydrate metabolic process; glycosphingolipid metabolic process; pathogenesis; keratan sulfate catabolic process; hyaluronan metabolic process; hyaluronan catabolic process Disease: Tay-sachs Disease |
| NCBI Summary: | This gene encodes the alpha subunit of the lysosomal enzyme beta-hexosaminidase that, together with the cofactor GM2 activator protein, catalyzes the degradation of the ganglioside GM2, and other molecules containing terminal N-acetyl hexosamines. Beta-hexosaminidase is composed of two subunits, alpha and beta, which are encoded by separate genes. Both beta-hexosaminidase alpha and beta subunits are members of family 20 of glycosyl hydrolases. Mutations in the alpha or beta subunit genes lead to an accumulation of GM2 ganglioside in neurons and neurodegenerative disorders termed the GM2 gangliosidoses. Alpha subunit gene mutations lead to Tay-Sachs disease (GM2-gangliosidosis type I). [provided by RefSeq, Jul 2009] |
| UniProt Code: | P06865 |
| NCBI GenInfo Identifier: | 311033393 |
| NCBI Gene ID: | 3073 |
| NCBI Accession: | P06865.2 |
| UniProt Secondary Accession: | P06865,Q53HS8, Q6AI32, B4DKE7, E7ENH7, |
| UniProt Related Accession: | P06865 |
| Molecular Weight: | 529 |
| NCBI Full Name: | Beta-hexosaminidase subunit alpha |
| NCBI Synonym Full Names: | hexosaminidase A (alpha polypeptide) |
| NCBI Official Symbol: | HEXA�� |
| NCBI Official Synonym Symbols: | TSD�� |
| NCBI Protein Information: | beta-hexosaminidase subunit alpha; hexosaminidase subunit A; beta-N-acetylhexosaminidase subunit alpha; N-acetyl-beta-glucosaminidase subunit alpha |
| UniProt Protein Name: | Beta-hexosaminidase subunit alpha |
| UniProt Synonym Protein Names: | Beta-N-acetylhexosaminidase subunit alpha; Hexosaminidase subunit A; N-acetyl-beta-glucosaminidase subunit alpha |
| Protein Family: | Hexamerin |
| UniProt Gene Name: | HEXA�� |
| UniProt Entry Name: | HEXA_HUMAN |