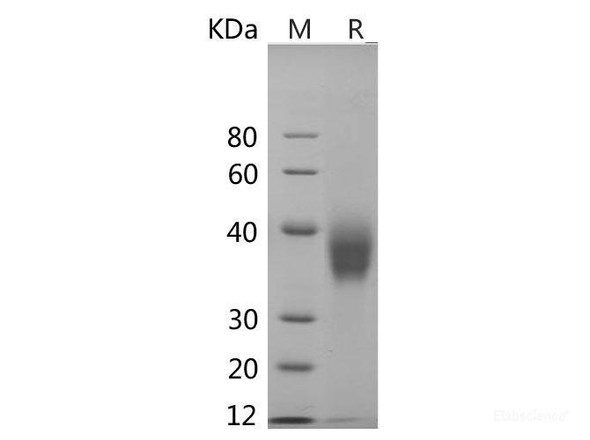
| Background: | Granulins are a family of secreted, glycosylated peptides that are cleaved from a single precursor protein with 7.5 repeats of a highly conserved 12-cysteine granulin/epithelin motif. The precursor protein, progranulin, is also called proepithelin and PC cell-derived growth factor. Cleavage of the signal peptide produces mature granulin which can be further cleaved into a variety of active, 6 kDa peptides. These smaller cleavage products are named granulin A, granulin B, granulin C, etc. Epithelins 1 and 2 are synonymous with granulins A and B, respectively. Both the peptides and intact granulin protein regulate cell growth. However, different members of the granulin protein family may act as inhibitors, stimulators, or have dual actions on cell growth. Granulin family members are important in normal development, wound healing, and tumorigenesis. Granulins have possible cytokine-like activity. They may play a role in inflammation, wound repair, and tissue remodeling. Granulin-4 promotes proliferation of the epithelial cell line A431 in culture while granulin-3 acts as an antagonist to granulin-4, inhibiting the growth. Granulin expression inhibited Tat transactivation, and tethering experiments showed that this effect was due, at least in part, to a direct action on cyclin T1 in the absence of Tat. |