Description
system_update_altDatasheet
| Product Name: | Human Cytokine 16-Plex Panel 2 |
| Product Code: | HUAMPM056 |
| Reactivity: | Human |
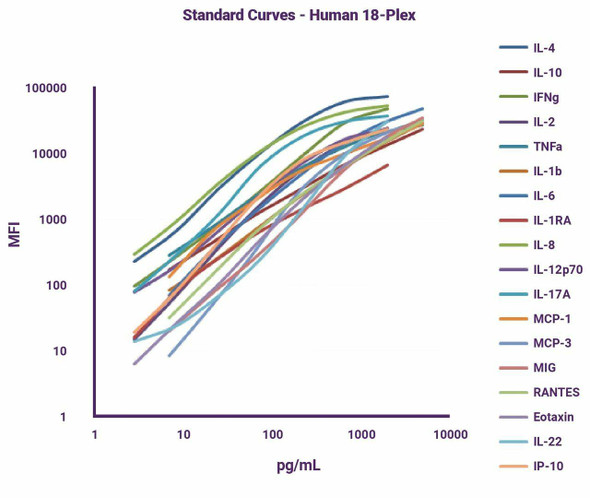
| Analytes: | CXCL2, GM-CSF, IFNalpha2, IFN?, IL-1beta, IL-1RA, IL-4, IL-6, IL-10, IL-12p70, IL-17A, IL-18, IL-21, IL-22, MCP-1, and TNFalpha |
| Sensitivity (LODs): | CXCL2: < 10 pg/mL, GM-CSF: < 5 pg/mL, IFNalpha2: < 5 pg/mL, IFNgamma: < 3 pg/mL, IL-1beta: < 5 pg/mL, IL-1RA: < 20 pg/mL, IL-4: < 1 pg/mL, IL-6: < 5 pg/mL, IL-10: < 2 pg/mL, IL-12p70: < 3 pg/mL, IL-17A: < 3 pg/mL, IL-18: < 5 pg/mL, IL-21: < 5 pg/mL, IL-22: < 3 pg/mL, MCP-1: < 2 pg/mL, TNFalpha: < 1 pg/mL. |
| Sample types: | Cell culture supernatant, serum, plasma, bodily fluid and tissue/cell lysate |
| Assay Type: | Multiplex |
| Detection Method: | Flow Cytometry |
| Assay Time: | 2 hours |
| 1x Ab-conjugated beads (premixed): | S4P2 - anti-H IL-1beta, S4P4 - anti-H IL-6, S4P6 - anti-H TNFalpha, S4P7 - anti-H IL-22, S4P8 - anti-H IL-1RA, S4P9 - anti-H IFNgamma, S4P11 - anti-H MCP-1, S4P12 - anti-H IL-12p70, S5P3 - anti-H GM-CSF, S5P4 - anti-H IL-4, S5P6 - anti-H CXCL2, S5P7 - anti-H IL-17A, S5P9 - anti-H IL-18, S5P10 - anti-H IL-10, S5P11 - anti-H IFNalpha2, S5P12 - anti-H IL-21 |
| 2x Biotin-detection Ab (premixed): | Biotin - anti-H IL-1beta, Biotin - anti-H IL-6, Biotin - anti-H TNFalpha, Biotin - anti-H IL-22, Biotin - anti-H IL-1RA, Biotin - anti-H IFNgamma, Biotin - anti-H MCP-1, Biotin - anti-H IL-12p70, Biotin - anti-H GM-CSF, Biotin - anti-H IL-4, Biotin - anti-H CXCL2, Biotin - anti-H IL-17A, Biotin - anti-H IL-18, Biotin - anti-H IL-10, Biotin - anti-H IFNalpha2, Biotin - anti-H IL-21 |
| Buffers: | 2x NR dAb Diluent. One vial containing 1.25 mL of biotin detection antibody diluent. |
| Lyophilized Standard Mix: | One vial containing lyophilized recombinant CXCL2, GM-CSF, IFN?2, IFN?, IL-1?, IL-1RA, IL-4, IL-6, IL-10, IL-12p70, IL-17A, IL-18, IL-21, IL-22, MCP-1, and TNF?. |
| LLOQs: | CXCL2: < 20 pg/mL, GM-CSF: < 10 pg/mL, IFNalpha2: < 10 pg/mL, IFN?: < 10 pg/mL, IL-1?: < 10 pg/mL, IL-1RA: < 40 pg/mL, IL-4: < 3 pg/mL, IL-6: < 10 pg/mL, IL-10: < 5 pg/mL, IL-12p70: < 10 pg/mL, IL-17A: < 10 pg/mL, IL-18: < 10 pg/mL, IL-21: < 10 pg/mL, IL-22: < 10 pg/mL, MCP-1: < 5 pg/mL, TNF?: < 2 pg/mL |
| ULOQs: | CXCL2: > 5,000 pg/mL, GM-CSF: > 5,000 pg/mL, IFNalpha2: > 5,000 pg/mL, IFN ?: > 2,000 pg/mL, IL-1?: > 5,000 pg/mL, IL-1RA: > 15,000 pg/mL, IL-4: > 1,000 pg/mL, IL-6: > 5,000 pg/mL, IL-10: > 5,000 pg/mL, IL-12p70: > 2,000 pg/mL, IL-17A: > 1,000 pg/mL, IL-18: > 5,000 pg/mL, IL-21: > 5,000 pg/mL, IL-22: > 2,000 pg/mL, MCP-1: > 5,000 pg/mL, TNF?: > 1,000 pg/mL |
| Standard dose recovery: | 70-130% |
| Intra-assay CV: | <10% |
| Inter-assay CV: | <20% |
| Cross-reactivity of analytes in the Panel: | Negligible |
| Sample volume: | 15 µL/test |
*Note: The below protocol is a sample protocol. Protocols are specific to each batch/lot. For the correct instructions please follow the protocol included in your kit.
A filter plate washer is required for the following protocol. For more information on GeniePlex click here.
| Step | Protocol |
| 1. | Prepare the filter plate template. Mark the standard, sample and blank wells. Standards and samples should be run in duplicates or triplicates. If the whole plate will not be used, seal the unused well with a plate seal. IMPORTANT: Place the filter plate on top of the filter plate lid during the entire assay process to prevent touching the plate bottom on any surface. |
| 2. | Vortex working bead suspension for 15 seconds. |
| 3. | Add 45 µ:L of capture bead working suspension to each well. NOTE: Save the remaining capture bead working suspension and store at 2-8°:C with light protection. It can be used for setting up acquisition parameters on the flow cytometer. |
| 4. | Remove buffer in the wells by using the "flow-through" Filter Plate Washer connected to a vacuum source that has been adjusted according to the Filter Plate Washer Instructions. |
| 5. | Remove buffer in the wells by using the "flow-through" Filter Plate Washer connected to a vacuum source that has been adjusted according to the Filter Plate Washer Instructions. |
| 6. | Add 30 µ:L of CCS, SPB or TL Assay Buffer to each sample well. NOTE: Cell culture supernatant samples can be run without diluting in Assay Buffer if very low levels (less than 20 pg/mL) of cytokines are expected. If it is the case, skip this step and add 45 µ:L of cell culture supernatant samples to each sample well in Step 7. |
| 7. | Add 15 µ:L of samples to each sample well. Add 45 µ:L of standards to each standard well. Cover the plate with a plate seal. |
| 8. | Incubate on the shaker (set at 700 rpm) for 60 min at room temperature. Protect from light by wrapping the filter plate in aluminum foil. |
| 9. | Remove the plate seal. Remove solutions in the wells by using the Filter Plate Washer connected to a vacuum source. |
| 10. | Remove solutions in the wells by using the Filter Plate Washer connected to a vacuum source. |
| 11. | Wash the wells three times with 100µ:L 1x Wash Buffer using the Filter Plate Washer. |
| 12. | Gently tap the plate bottom onto several layers of paper towels to remove residual buffer on the plate bottom after the last wash. |
| 13. | Add 25µ:L of biotinylated antibody working solution to each well. Cover the plate with a plate seal. |
| 14. | Incubate on the shaker (set at 700 rpm) for 30 min at room temperature. Protect from light by wrapping the filter plate in aluminum foil. |
| 15. | Remove the plate seal. Remove solutions in the wells by using the Filter Plate Washer. Wash the wells three times with 100 µ:L 1x Wash Buffer using the Filter Plate Washer. |
| 16. | Gently tap the plate bottom onto several layers of paper towels to remove residual buffer on the plate bottom after the last wash. |
| 17. | Add 25µ:L of streptavidin-PE working solution to each well. Cover the plate with a plate seal. |
| 18. | Incubate on the shaker (set at 700 rpm) for 20 min at room temperature. Protect from light by wrapping the filter plate in aluminum foil. |
| 19. | Remove the plate seal. Remove solutions in the wells by using the Filter Plate Washer. Wash the wells twice with 100 µ:L 1x Wash Buffer. |
| 20. | Gently tap the plate bottom onto several layers of paper towels to remove residual solution. Add 150µ:L to 300µ:L of 1x Reading Buffer to each well depending on the sample loading mechanism of a flow cytometer to re-suspend the beads. Cover the plate with a plate seal. |
| 21. | Place the plate on the microtiter shaker and shake for 30 seconds at 700 rpm. NOTE: If the flow cytometer has no 96-well plate loader and more than 200 µ:L of 1x Reading Buffer is needed to re-suspend the beads, do not shake the plate. Re-suspend the beads in each well by pipetting up and down 68 times with a P200 pipette then transfer to a test tube for acquisition. Remove the plate seal. Read on a flow cytometer. |
GeniePlex Technology
GeniePlex uses a unique mix of antibody-coated encoded microparticles providing an ultra-sensitive technology for the quantitation of analytes.
At Assay Genie we understand the need for quantitative reproducible results! Therefore, we have developed a simplified protocol meaning you can easily quantify up to 24 analytes in as little as 15µ:L of sample all in 32-test or 96-test filter plate format for rapid analysis.