Description
GenieHTS Multidrug Resistance (MDR) Activity Assay
GenieHTS MDR Activity Assay kit is an effective solution for detecting MDR1 and MRP1 activity and compounds susceptible to MDR-mediated efflux. GenieHTS MDR Activity Assay is compatible with fluorescence microscopy, flow cytometry, and fluorescence plate readers using FITC/GFP settings.
| Product Name: | GenieHTS MDR Activity Assay Kit |
| Product Code: | ASIB007 |
| Product Size: | 10 plates |
| Excitation: | 495nm |
| Emission: | 515nm |
| Component Name | Size | Storage |
| Calcein AM in DMSO (4 mM) | 50 μL x 2 | -20°C |
| 1000x Cyclosporine A in Ethanol | 50 μL | -20°C |
| 1X Assay Buffer, pH 7.3 | 100 mL | RT |
| TRS | 4 mL | RT |
Materials needed but not provided
- Compounds to be tested.
- Buffers and solvents for dissolution.
- Reagents necessary for cell culture.
- A microscope
- A fluorescence plate reader ~ 490 nm /~ 520 nM.
- Compatible filters are FITC or GFP.
Assay Genies' GenieHTS MDR Activity Assay Kit is an effective solution for detecting MDR1 and MRP1 activity and compounds susceptible to MDR-mediated efflux. The GenieHTS MDR Activity kit is compatible with fluorescence microscopy, flow cytometry, and fluorescence plate readers using FITC/fluorescein settings. Calcein AM is a membrane-permeant, non-fluorescent dye that enters cells passively. Once inside the cytosol of cells, intracellular esterases convert it to fluorescent Calcein (Ex/Em: 495 nm/515 nm), resulting in uniform cytosolic fluorescence. Drug efflux transporters, such as P-glycoprotein (Pgp, MDR1) and multidrug-resistance- associated protein (MRP1), actively extrude Calcein AM from inside the cell before esterases can convert it to Calcein.
The presence of additional MDR substrates or inhibitors of MDR expression results in decreased Calcein AM efflux, causing a measurable increase in intracellular fluorescence. In addition to identifying MDR substrates and inhibitors, this kit can also be used to evaluate the activity of MDR transporters in cells. When following our protocol, Assay Genies' GenieHTS MDR Activity Kit provides enough reagents to make 100 mL of working solution, enough for ten 96- or 384-well plates or 80 flow cytometry samples. The actual number of assays will vary according to optimal dye concentrations for your application.
Assay Notes
- Optimal Calcein AM concentrations will vary depending on cell type and application. Recommended Calcein AM concentrations range between 1 μM and 2 μM.
- Minimize freeze-thaw cycles of Calcein AM and Cyclosporine A solutions.
- Aqueous solutions of Calcein AM are susceptible to hydrolysis; therefore, all working solutions should be used as quickly as possible and no later than 4 hours after preparation.
- Calcein cannot withstand fixation after staining.
- Serum-containing solutions may increase extracellular fluorescence. If conducting an assay with serum present, we recommend including TRS to minimize extracellular fluorescence.
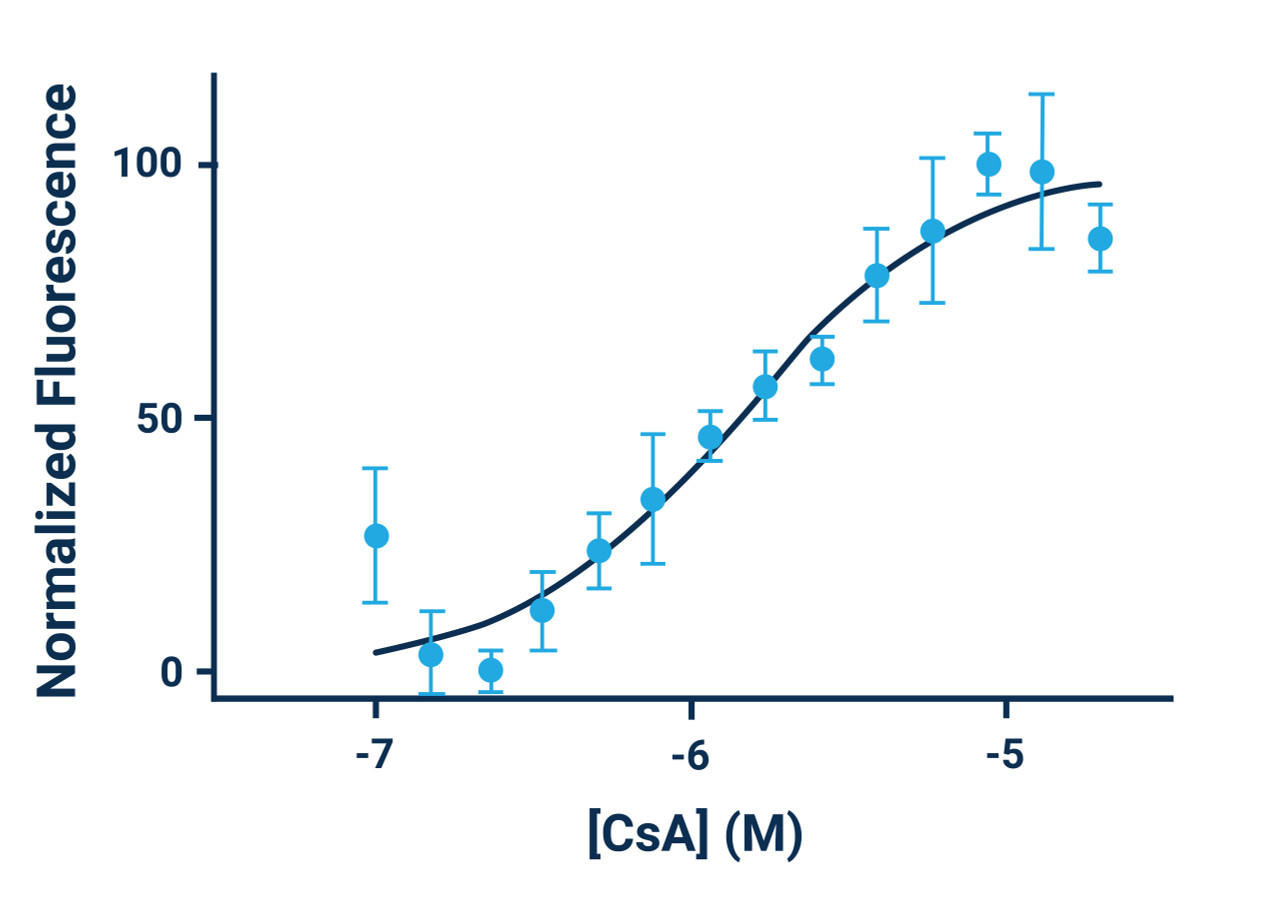
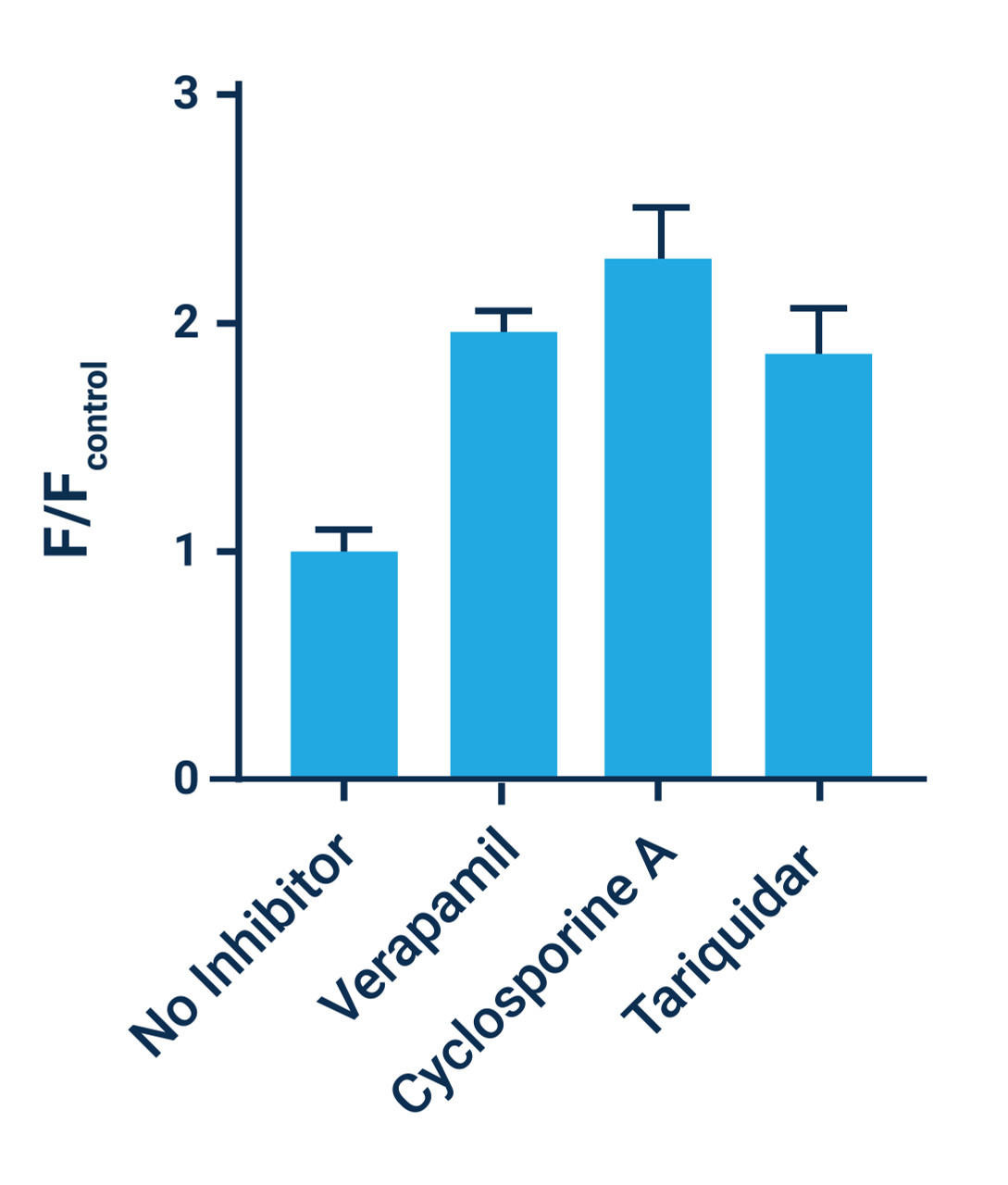
- Cyclosporine A can be used as a positive control. Use at a concentration of 5 μM (1:1000 dilution) for maximum efficacy.
- Molecules that affect esterase activity, intracellular ATP production, or that bind to alternative MDR binding sites may result in an inaccurate classification of MDR substrates.
Assay Procedure
Plate Reader
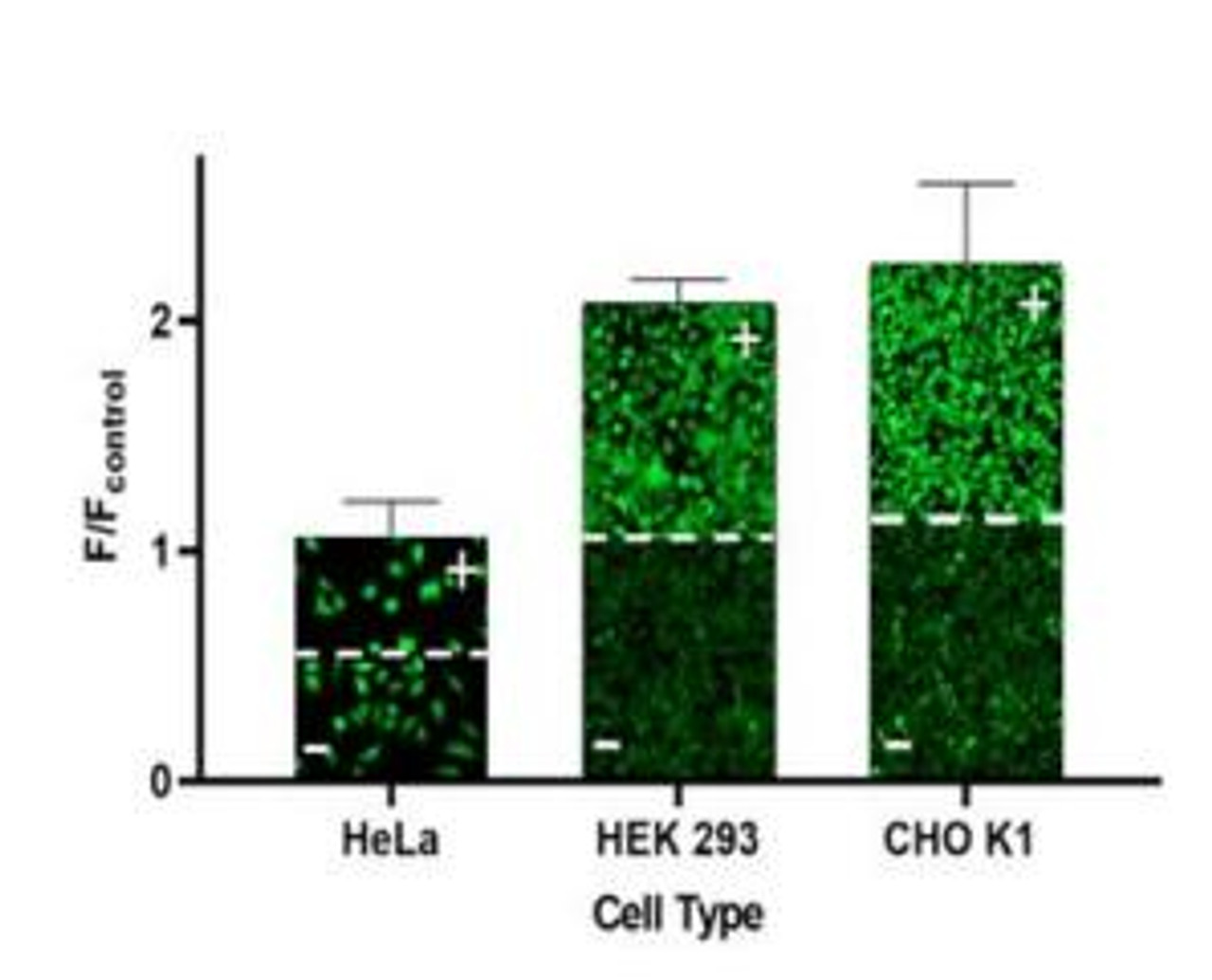
- For adherent cells, plate cells overnight, seed ~30,000 cells/well/100 μL in a 96-well plate or ~10,000 cells/well/25 μL in a 384-well plate. When using this assay kit, make sure to use cells that possess high levels of MDR activity such as CHO K1, HCT-8, U2OS, or cells that have been genetically engineered to overexpress MDR proteins.
- Remove one vial of Calcein AM and 1000X Cyclosporine A from freezer and allow to warm to room temperature. Protect reagents from light.
- Prepare the test compound solutions at the desired concentrations in buffer of choice (HBSS or HHBSS).
- For a positive control, make the appropriate amount of Cyclosporine A solution needed, enough for 100 μL/well to be evaluated. For example, add 1 μL of 1000X Cyclosporine A solution to 0.999 mL of the buffer used in step 3.
- Remove medium from the wells of the plate and add 100 μL/well of test compound solutions, including your positive control solution from step 4. Incubate for 15 - 30 minutes at 37°C or room temperature.
- Prepare dye loading solution that contains 2X Calcein AM, 4 μM (1:1000 dilution) in 1X Assay Buffer. For example, add 10 μL of Calcein AM to 10 mL of 1X Assay Buffer for a full plate. Vortex briefly to mix.
- Optional: Add 400 μL of TRS to the dye loading solution. Adjust 1X Assay Buffer volume in step 6 to 9.6 mL. TRS minimizes extracellular fluorescence and is recommended when long incubation times with dye loading solution or serum containing media are used.
- After the 15 - 30 minute incubation period from step 5 is complete, add dye loading solution prepared in step 6/7 directly to wells. Add 100 μL/well to a 96-well plate.
- Incubate cells for an additional 30 minutes at 37 °C or room temperature. Protect from light.
- Measure fluorescence using a fluorescence plate reader. For Calcein, use Ex/Em ~495 nm/515 nm or FITC settings.
*Note: If conducting an assay using a fluorescence microscope, use GFP or FITC filters to acquire images. If using non- adherent cells, follow flow cytometry protocol until step 10. Then add cell suspensions to wells of a microplate and centrifuge your plate before acquiring fluorescence data.
Flow Cytometry
- Remove one vial of Calcein AM and 1000X Cyclosporine A from freezer and allow to warm to room temperature.
- Protect reagents from light.
- Prepare your test compounds at the desired concentrations in 1 mL of the buffer of your choice (HBSS or HHBSS). To prepare the positive control, add 1 μL of the 1000X Cyclosporine A solution to 0.999 mL of the same buffer.
- If using adherent cells, detach cells from the culture dish and suspend cells in cell culture medium at a concentration of ~1-2 x 106 cells/mL. For non-adherent cells, suspend in medium at your desired cell concentration.
- Prepare tubes for each assay condition in triplicate by adding 250 μL of the cell suspension from step 3 to a minimum of 3 tubes. Add an extra tube for an unstained cell control, if desired.
- Centrifuge cells and remove the medium.
- Resuspend a single tube of cells in 125 μL of test compound solution or positive control solution. Repeat in triplicate (3 tubes total) for each test compound. Also include a vehicle control condition of 125 μL of your buffer containing the same DMSO concentration as your test compound solutions.
- Incubate tubes for 15 - 30 minutes at room temperature.
- During incubation period, prepare a dye loading solution that contains 2X Calcein AM, 4 μM (1:1000 dilution) in 1X Assay Buffer. For example, add 10 μL of Calcein AM to 10 mL of 1X Assay Buffer. Vortex briefly to mix.
- After the 15 - 30 minute incubation period from step 7 is complete, add 125 μL of dye loading solution prepared in step 8 to each tube.
- Incubate cells for an additional 30 minutes at room temperature. Protect from light.
- After the completion of step 10, centrifuge cells and aspirate your dye loading solution and test compound buffer. Resuspend cells in your preferred flow cytometry (FACS) buffer.