GenieHTS Live or Dead Assay Kit
- SKU:
- ASIB008
- Product Type:
- Assay
- Detection Method:
- Fluorometric
- Sample Type:
- Cell Samples
- Research Area:
- Apoptosis
Description
GenieHTS Live or Dead Assay Kit
Two-color, fluorescence-based assay to discriminate between live and dead cells
| Product Name: | GenieHTS Live or Dead Assay Kit |
| Product Code: | ASIB008 |
| Product Size: | 15 plates |
| Excitation: | Live 495nm, Dead 528nm |
| Emission: | Live 515nm, Dead 617nm |
| Component Name | Size | Storage |
| Calcein AM in DMSO (4Mm) | 100 μL | -20°C |
| Ethidium homodimer I (EthD-I) in DMSO/H2O (2mM) | 300 μL | -20°C |
Materials needed but not provided
- Compounds to be tested.
- Buffers and solvents for dissolution.
- Reagents necessary for cell culture.
- A fluorescence plate reader ~ 490 nm /~ 520 nM and measuring emission at ~530 nm and ~620 nm
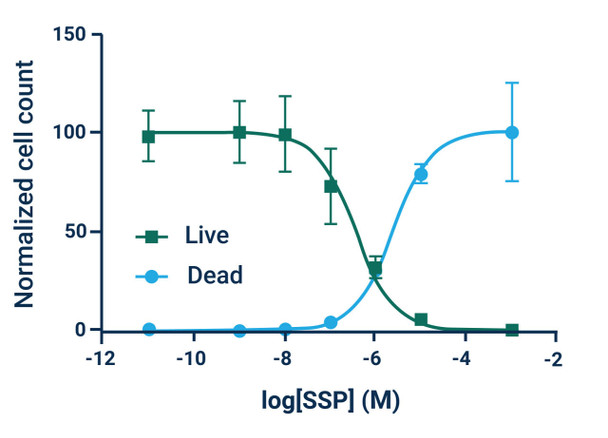
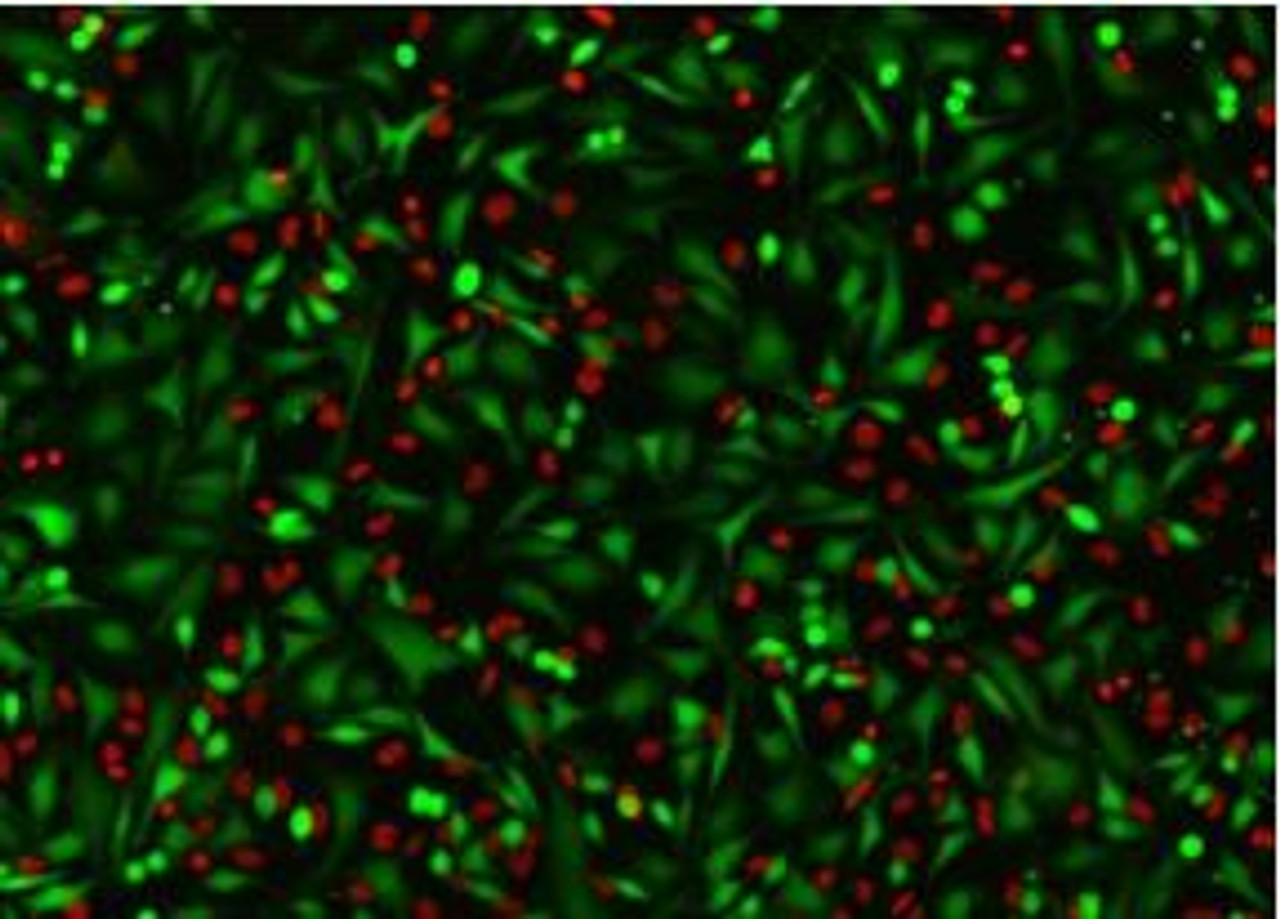
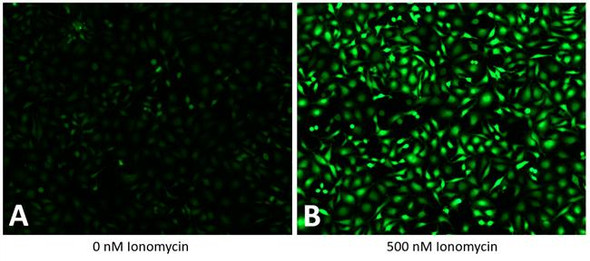
The Assay Genie Live or Dead assay kit is a simple, rapid, and versatile solution for detecting live (green) and dead (red) cells within a population. GenieHTS Live or Dead Assay is compatible with fluorescence microscopy, flow cytometry, and plate reader applications. Calcein AM is a membrane-permeant, non-fluorescent form of calcein that enters cells passively. Once inside the cytosol of cells, Calcein AM is converted to green-fluorescent Calcein by ubiquitous esterases in viable cells, resulting in uniform cytosolic fluorescence (Ex/Em 495 nm/515 nm). Calcein, a polyanionic dye, is membrane- impermeant and is well retained with in the cytosol of healthy cells with intact cell membranes.
Ethidium homodimer I (EthD-I) is a membrane-impermeable, high-affinity, nucleic acid stain that is excluded by viable cells with intact cell membranes. When membrane integrity is compromised, a hallmark of dead or dying cells, EthD-I enters the cell and binds DNA, which results in a >30-fold enhancement in bright red, nuclear fluorescence (Ex/Em 528 nm/617 nm). GenieHTS Live or Dead Assay can be used to quantify live and dead cells within eukaryotic cell suspensions or adherent cultures, 3D cultures, organoids, and some non-fixed tissues, but cannot be used for yeast or bacteria. When following our protocol, GenieHTS Live or Dead Assay kit provides enough reagents to make 150 mL of working solution, enough for fifteen 96-well plates or 1,500 flow cytometry samples. The actual number of assays will vary according to optimal dye concentrations for your application.
Assay Notes
- Optimal dye concentrations will vary depending on cell type and application. Recommended dye concentrations range between 0.1 μM and 10 μM.
- Aqueous solutions of Calcein AM are susceptible to hydrolysis; therefore, all working solutions should be used as quickly as possible and no later than 24 hours after preparation.
- Serum-containing preparations will increase extracellular Calcein fluorescence. When possible, A wash step can remove extracellular fluorescence.
- Calcein cannot withstand fixation after staining.
- Dead cell controls can be prepared by applying 0.1% saponin or 0.1 - 0.5% digitonin to live cells for 10 min.
- Cytotoxic events that do not affect esterase activity or membrane permeability are not going to be accurately measured using this kit.
Assay Procedure
Plate Reader Assay
- Seed cells in a 96-well (or 384-well) plate and treat with test compounds of your choosing prior to staining.
- Remove stock solutions of Calcein AM and EthD-I from freezer and allow to warm to room temperature.
- Prepare dead cells by treating with 0.1% saponin or digitonin solutions for 10 min.
- Prepare a working solution that contains 2 μM Calcein AM (1:2000 dilution) and 4 μM EthD-I (1:500 dilution) in phosphate buffered saline (PBS) or other serum free medium or buffer. For example, add 5 μL of Calcein AM stock and 20 μL of EthD-I stock to 10 mL of PBS. Vortex briefly to mix.
- Optional: Wash the cells with serum-free buffer or medium to remove serum. For suspension cells, use a centrifuge to pellet cells, then resuspend in 100 μL of serum free medium or buffer. The wash solution can be aspirated from wells prior to the addition of working solution if desired.
- Add working solution prepared in step 4 directly to cells. We recommend 100 μL/well for a 96-well plate.
- Incubate cells for 30 - 45 min at room temperature or 37 °C. Protect from light.
- Measure fluorescence using a microplate reader. For Calcein, use Ex/Em ~485 nm/520 nm or FITC settings. For EthD-I, use Ex/Em ~530 nm/620 nm or Texas Red® settings.
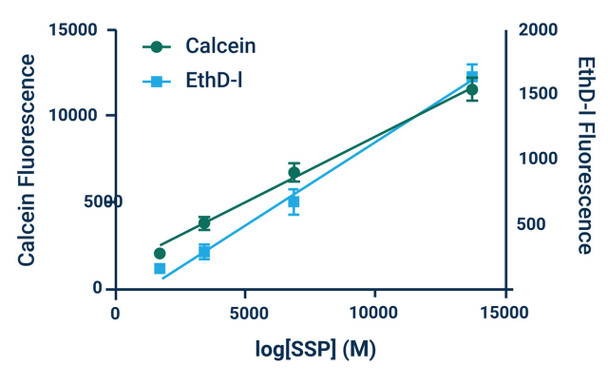
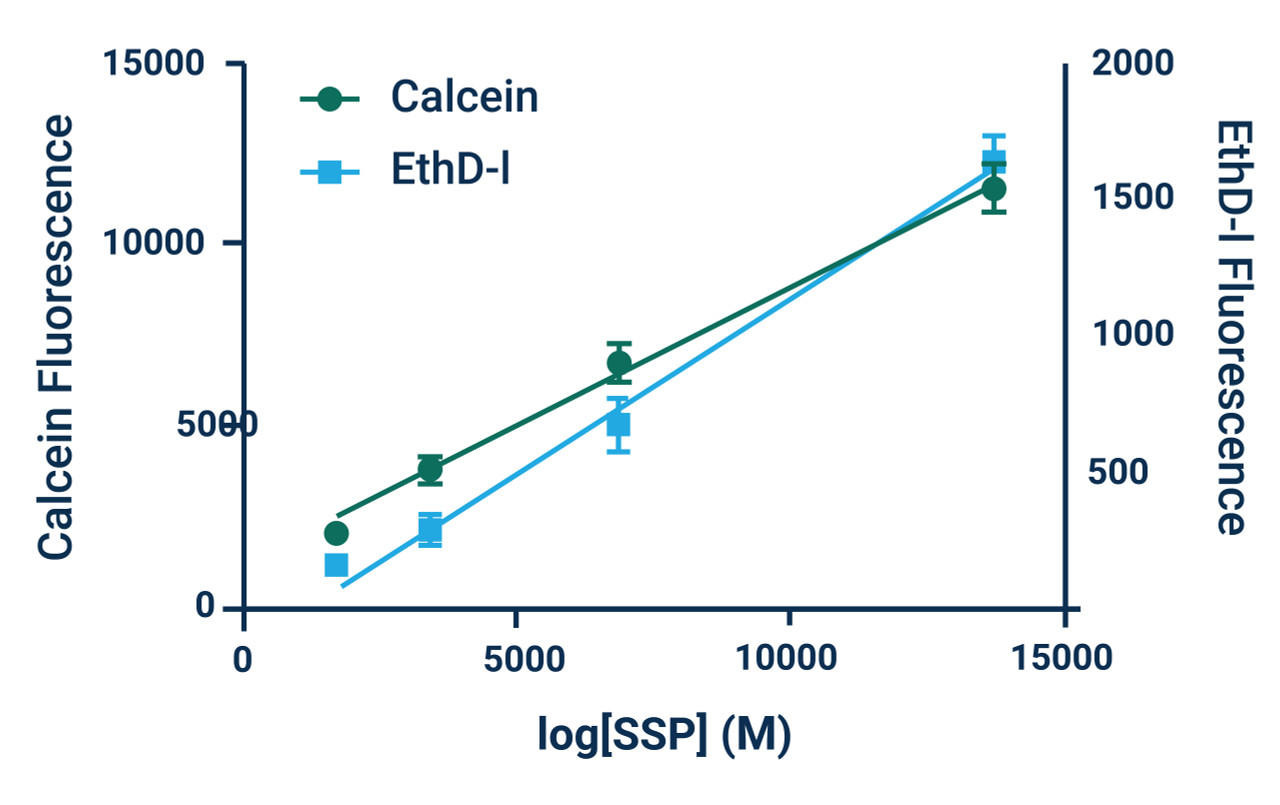
*Note: Absolute numbers of viable and dead cells can be estimated by generating standard curves. Standard curves are prepared by seeding known densities of healthy and dead cells in wells prior to staining. Standard curves should be created for both viable and dead cells. Dye fluorescence intensity is linearly related to the number of live or dead cells as demonstrated in Example Results.
Microscopy Assay
- Remove stock solutions of Calcein AM and EthD-I from freezer and allow to warm to room temperature.
- Protect reagents from light.
- Prepare a working solution that contains 2 μM Calcein AM (1:2000 dilution) and 4 μM EthD-I (1:500 dilution) in phosphate buffered saline (PBS) or other serum free medium or buffer. For example, add 5 μL of Calcein AM stock and 20μL of EthD-I stock to 10 mL of PBS. Vortex briefly to mix.
- Optional: Wash the cells with serum-free buffer or medium to remove serum.
- Add sufficient volume of the working solution prepared in step 2 to completely cover cells.
- Incubate cells for 30 - 45 min at room temperature or 37C. Protect from light.
- Optional: Replace working solution with fresh buffer or medium prior to imaging.
- Image stained cells using fluorescence microscopy. For Calcein, use a GFP or FITC filters. For EthD-I, use a Texas Red®, propidium iodide (PI), or rhodamine filters.
Flow Cytometry Assay
- Remove stock solutions of Calcein AM and EthD-I from freezer and allow to warm to room temperature.
- Protect reagents from light.
- Prepare a working solution that contains 2 μM Calcein AM (1:2000 dilution) and 4 μM EthD-I (1:500 dilution) in phosphate buffered saline (PBS) or other serum free medium or buffer. For example, add 5 μL of Calcein AM stock and 20 μL of EthD-I stock to 10 mL of PBS. Vortex briefly to mix.
- Pellet cells via centrifugation, remove supernatant, then resuspend in 100 μL of serum free medium or buffer.
- Add 100 μL of working solution prepared in step 2 directly to cells.
- Incubate cells for 30 - 45 min at room temperature or 37C. Protect from light.
- Pellet cells again via centrifugation and resuspend in preferred flow cytometry buffer.
- Analyze cells using a flow cytometer. To detect calcein (+) cells, use FITC settings. To detect EthD-I (+) cells, use phycoerythrin (PE) or PE-Texas Red® settings. Use single color stained cells to perform standard compensation.