gelE Antibody (PACO50726)
- SKU:
- PACO50726
- Product Type:
- Antibody
- Reactivity:
- Bacteria
- Host Species:
- Rabbit
- Isotype:
- IgG
- Applications:
- ELISA
- WB
- Antibody Type:
- Polyclonal Antibody
- Conjugation:
- Unconjugated
Frequently bought together:
Description
| Antibody Name: | gelE Antibody (PACO50726) |
| Antibody SKU: | PACO50726 |
| Size: | 50ug |
| Host Species: | Rabbit |
| Tested Applications: | ELISA, WB |
| Recommended Dilutions: | ELISA:1:2000-1:10000, WB:1:500-1:5000 |
| Species Reactivity: | Enterococcus faecalis |
| Immunogen: | Recombinant Enterococcus faecalis Gelatinase protein (193-510AA) |
| Form: | Liquid |
| Storage Buffer: | Preservative: 0.03% Proclin 300 Constituents: 50% Glycerol, 0.01M PBS, pH 7.4 |
| Purification Method: | >95%, Protein G purified |
| Clonality: | Polyclonal |
| Isotype: | IgG |
| Conjugate: | Non-conjugated |
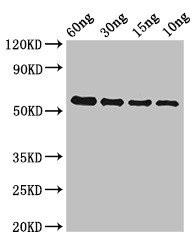 | Western Blot. Positive WB detected in Recombinant protein. All lanes: gelE antibody at 2µg/ml. Secondary. Goat polyclonal to rabbit IgG at 1/50000 dilution. predicted band size: 51 kDa. observed band size: 51 kDa.. |
| Background: | Metalloprotease capable of the hydrolysis of insoluble hydrophobic substrates. Hydrolyzes azocoll and gelatin and, at a lower rate, soluble and insoluble collagens. Does not cleave short synthetic peptides. Preferentially hydrolyzes the 24-Phe- |-Phe-25 bond in the insulin B-chain, followed by the 5-His- |-Leu-6 bond. Inactivates endothelin-1, primarily by cleavage of the 5-Ser- |-Leu-6 and 16-His- |-Leu-17 bonds. Hydrolyzes the α chain of C3 to generate a C3b-like protein. Inhibits complement-mediated hemolysis and opsinization of bacteria. Hydrolyzes the insect antimicrobial peptide cecropin. Decreases the length of E.faecalis chains via the activation of autolysin. Degrades polymerized fibrin. |
| Synonyms: | Gelatinase (EC 3.4.24.30) (Coccolysin), gelE |
| UniProt Protein Function: | Metalloprotease capable of the hydrolysis of insoluble hydrophobic substrates. Hydrolyzes azocoll and gelatin and, at a lower rate, soluble and insoluble collagens. Does not cleave short synthetic peptides. Preferentially hydrolyzes the 24-Phe-|-Phe-25 bond in the insulin B-chain, followed by the 5-His-|-Leu-6 bond. Inactivates endothelin-1, primarily by cleavage of the 5-Ser-|-Leu-6 and 16-His-|-Leu-17 bonds. Hydrolyzes the alpha chain of C3 to generate a C3b-like protein. Inhibits complement-mediated hemolysis and opsinization of bacteria. Hydrolyzes the insect antimicrobial peptide cecropin. Decreases the length of E.faecalis chains via the activation of autolysin. Degrades polymerized fibrin. |
| UniProt Protein Details: | |
| NCBI Summary: | |
| UniProt Code: | Q833V7 |
| NCBI GenInfo Identifier: | 29376362 |
| NCBI Gene ID: | 1200704 |
| NCBI Accession: | NP_815516.1 |
| UniProt Secondary Accession: | Q833V7,Q47786, Q9S385, A1E464, B5AN27 |
| UniProt Related Accession: | Q833V7 |
| Molecular Weight: | 50.5 kDa |
| NCBI Full Name: | coccolysin |
| NCBI Synonym Full Names: | |
| NCBI Official Symbol: | EF1818 |
| NCBI Official Synonym Symbols: | |
| NCBI Protein Information: | coccolysin |
| UniProt Protein Name: | Gelatinase |
| UniProt Synonym Protein Names: | Coccolysin |
| Protein Family: | Gelatinase |
| UniProt Gene Name: | gelE |
| UniProt Entry Name: | GELE_ENTFA |

