Therapeutic Antibody & Biosimilar ELISA Kits
Therapeutic Antibody & Biosimilar ELISA Kits
Quantitative and Qualitative Free Drug and ADA ELISA for Biologics & Biosimilars

Why choose ELISA for Drug Monitoring?
- Validated | For Cancer, Autoimmune diseases, Osteoporosis, Macular Degeneration research & more
- Screening | Qualitative, quantitative & free/total kits for full screening of antibodies.
- High Sensitivity | Sensitivity and limit of detection in the ng/mL range.
- Sample types | Validated in serum and/or plasma from human, mouse & NHP samples

Our TDM & ADA ELISA Range
Testimonials & Partners
"Assay Genie provided a critical ELISA kit that is very difficult to find on the market. Not only did this kit work dependably well, but it also provided me with the measurement sensitivity that I needed to evaluate my samples."
- Erika on Trustpilot


Citations
Product | Author | Journal | Citation |
|---|---|---|---|
Sensi et al. | Chemical Communications | ||
Gandhi et al. | Cancer Chemotherapy and Pharmacology | ||
Ahmed et al. | Farmacia |
cGMP & ISO Certification


Quality is at the core of everything we do.
Manufactured in state-of-the-art facilities, our products meet the highest standards, including current Good Manufacturing Practicies) cGMP, ISO 9001:2015, and ISO 13485:2016 certifications. These ensure consistent quality, customer-focused innovation, and reliable performance in every batch.
ELISA Manufacture Pipeline

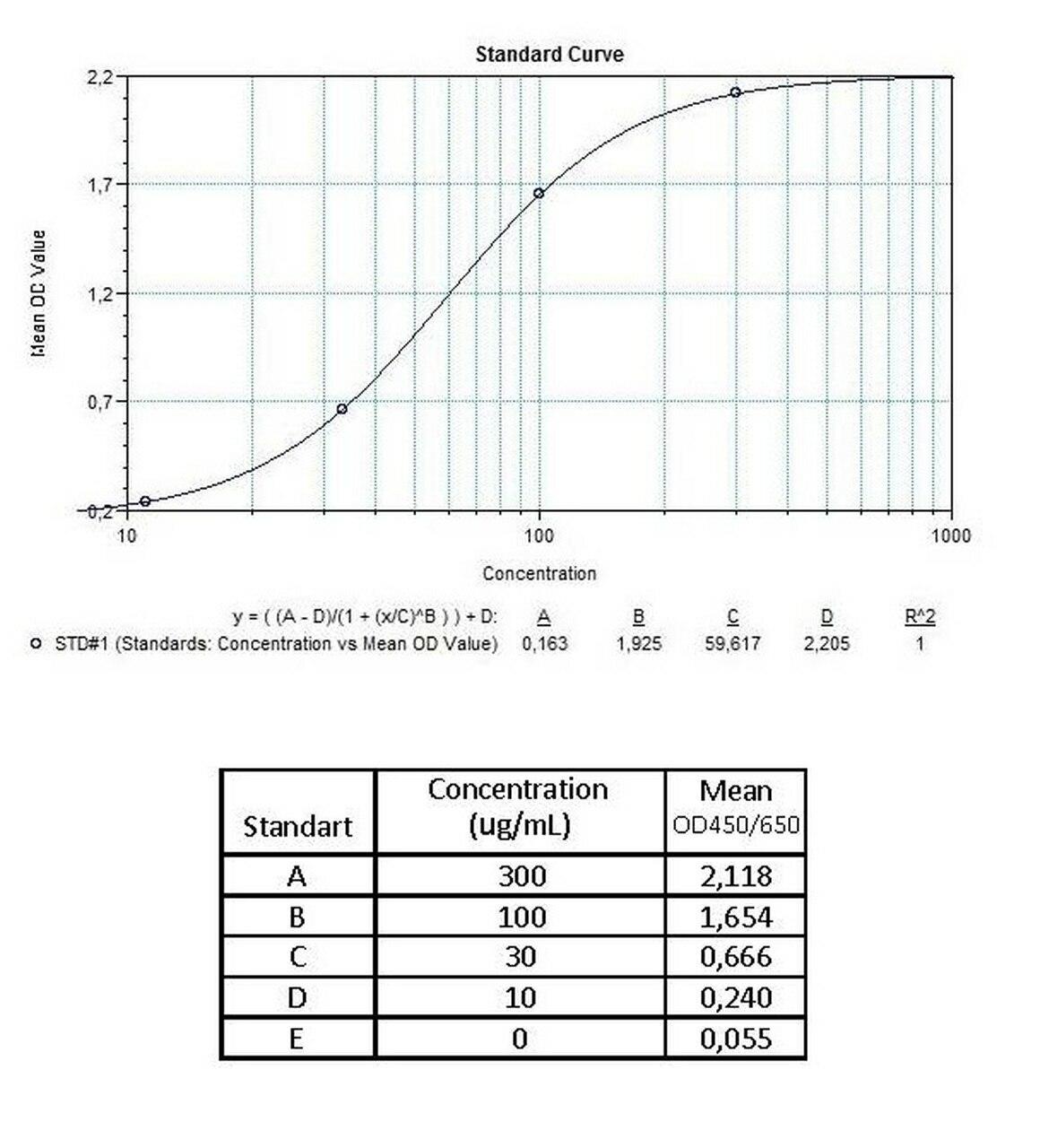
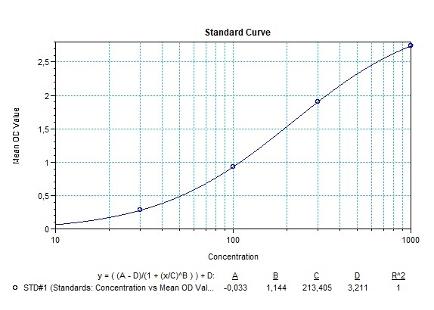
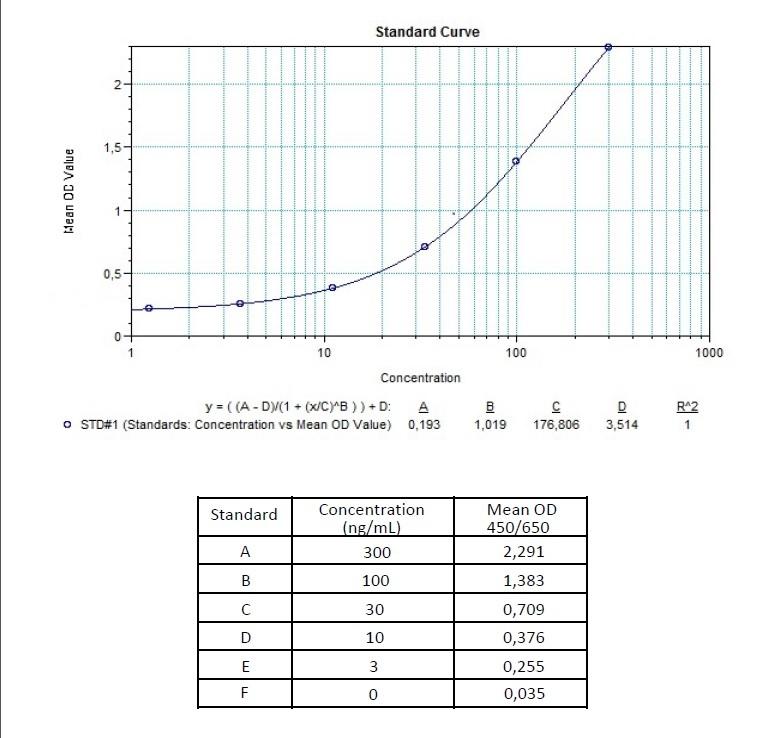
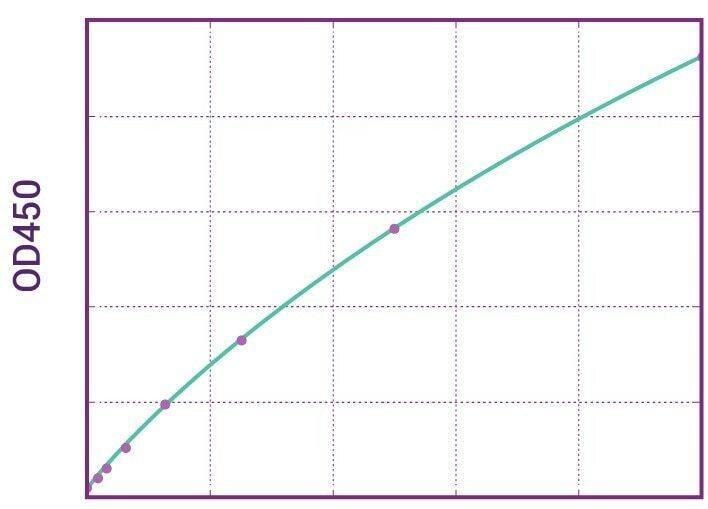
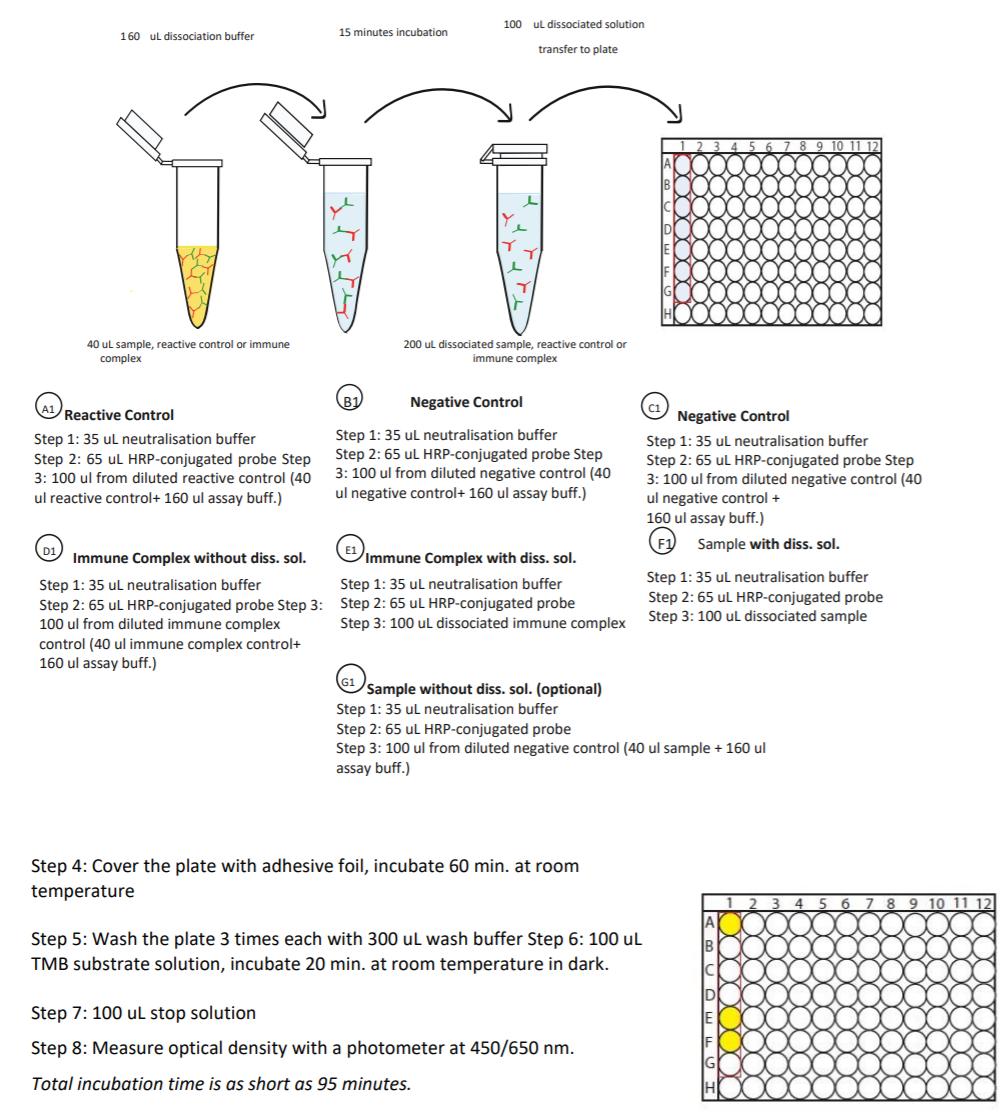
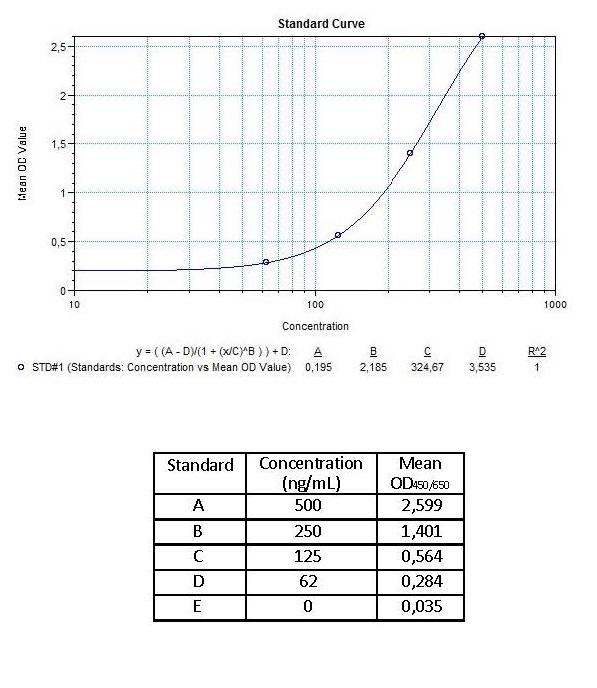
Detection Principle & Protocol
An ELISA protocol involves coating a microplate with a specific capture antibody, adding the sample containing the target analyte, and incubating to allow binding. After washing to remove unbound substances, a detection antibody (linked to an enzyme) is added, followed by a substrate solution that produces a measurable color change. The optical density (OD) is read using a spectrophotometer, with absorbance values correlating to analyte concentration.

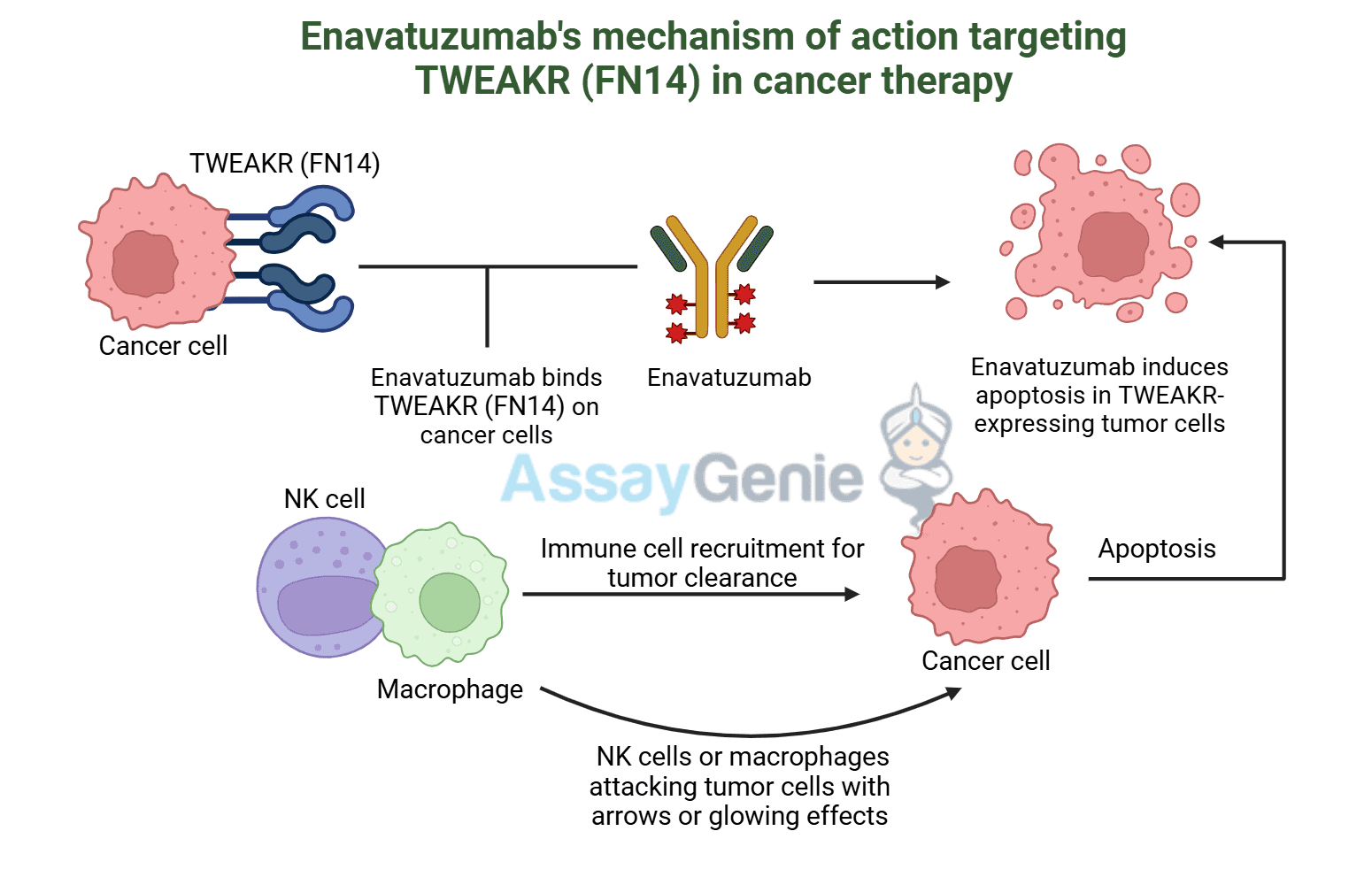
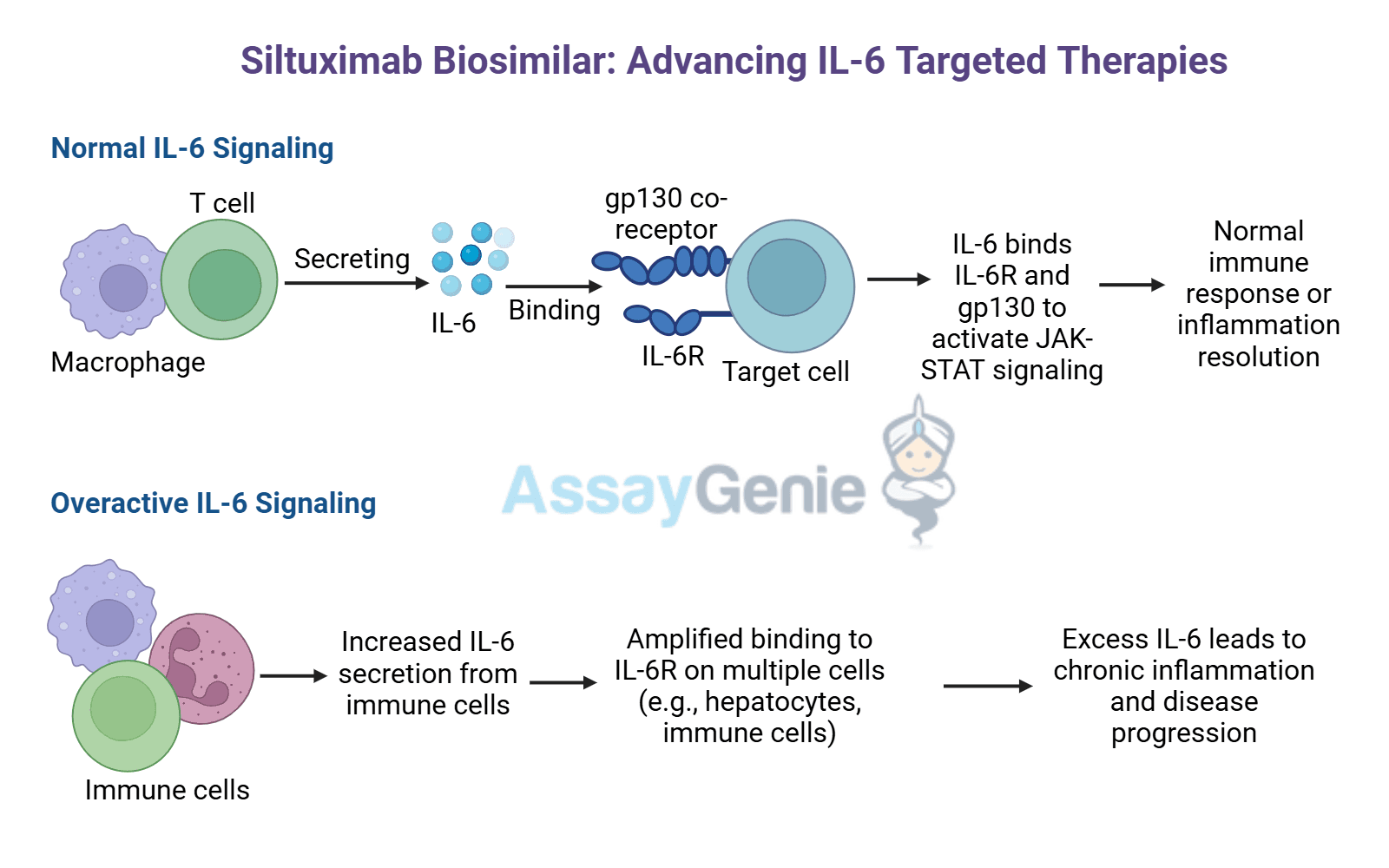
What are Therapeutic Antibodies & ADAs?
- Therapeutic mAbs | Designed to bind specific targets and modulate the immune responses or block disease-related pathways.
- Biosimilars | Highly similar versions of approved therapeutic mAbs, while offering a more cost-effective alternative.
- Anti-Drug Antibodies (ADAs) | Immune-generated antibodies that can bind or neutralize therapeutic mAbs, reducing effectiveness.

Why is drug monitoring important ?

- Optimized Dosing | Ensures therapeutic drug concentrations remain within the optimal range for maximum efficacy.
- Reduced Immunogenicity | Prevents overdosing-related side effects and identifies ADA formation that may reduce drug effectiveness.
- Personalized Treatment | Enables dose adjustments based on individual patient responses, improving long-term outcomes
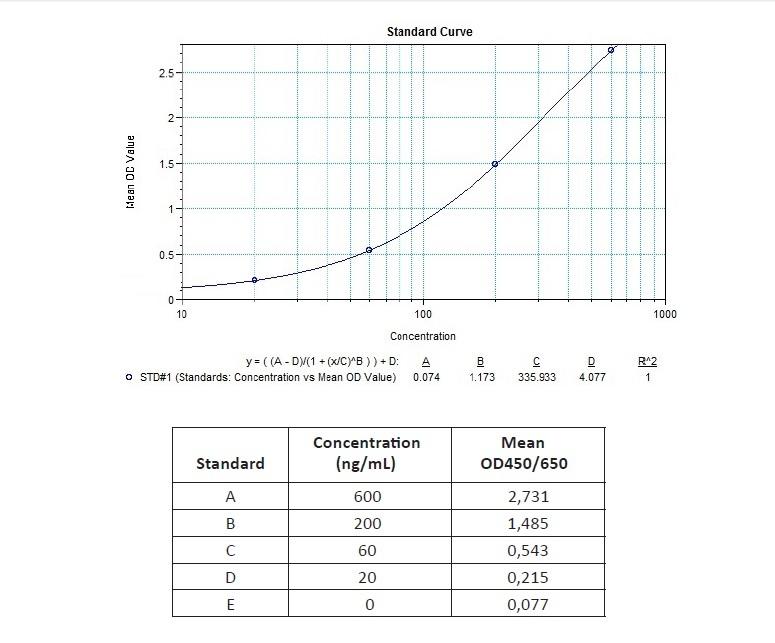
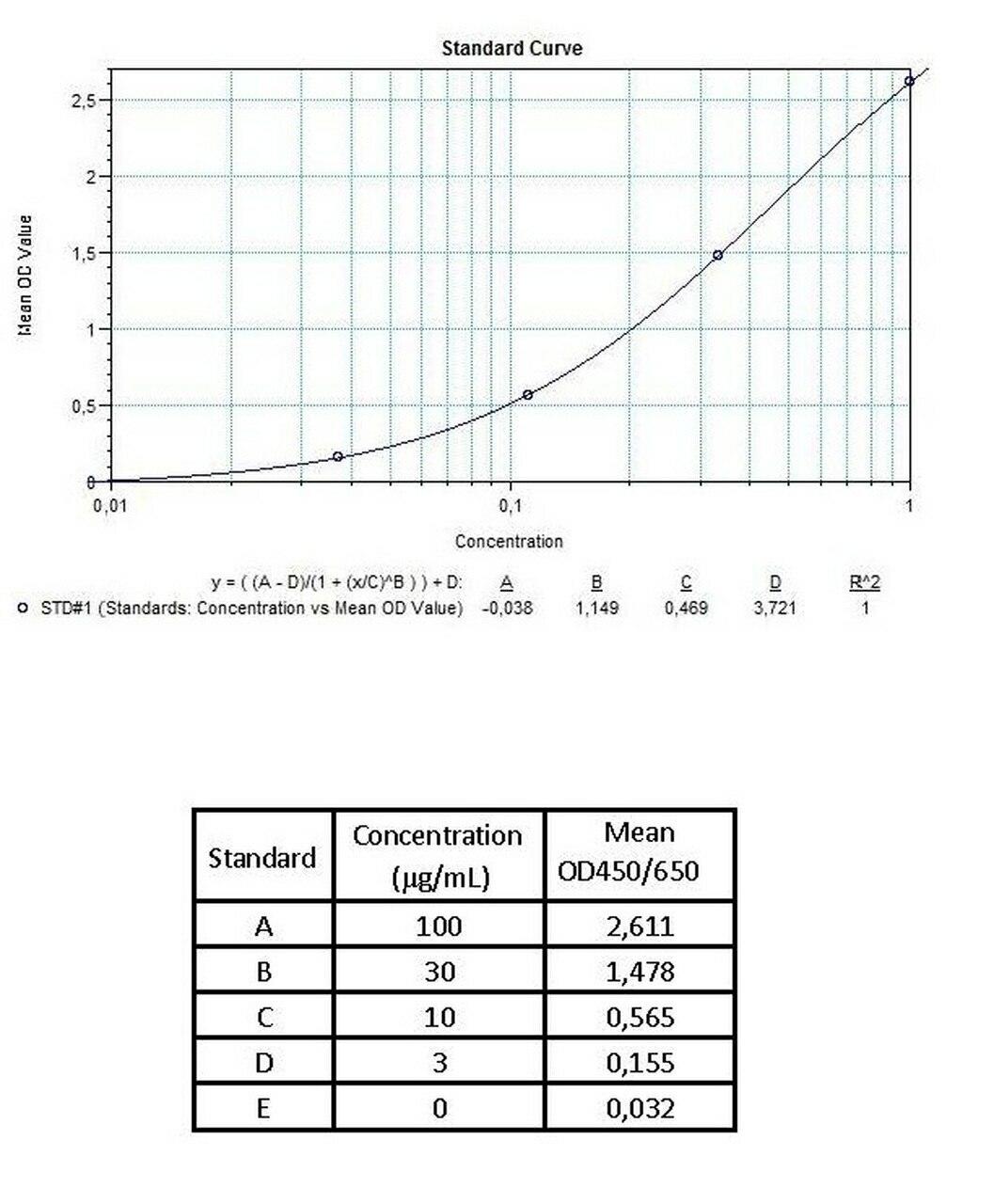
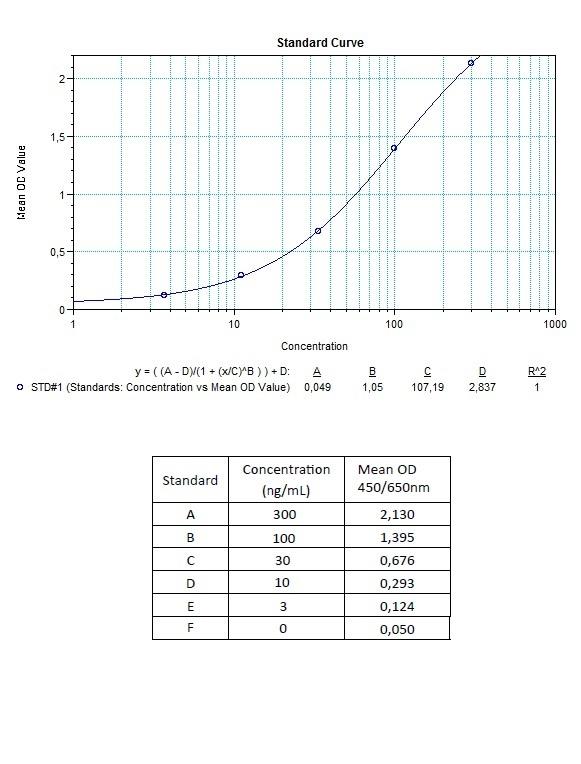
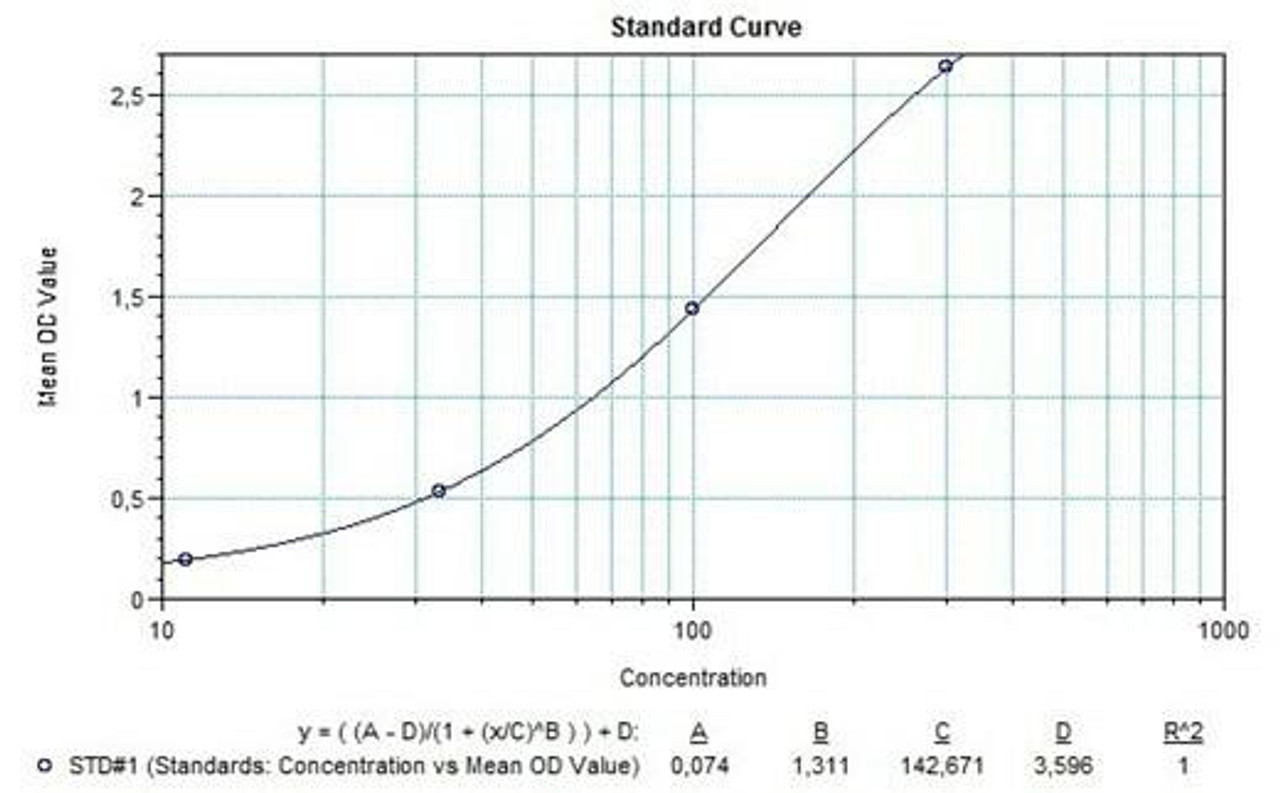
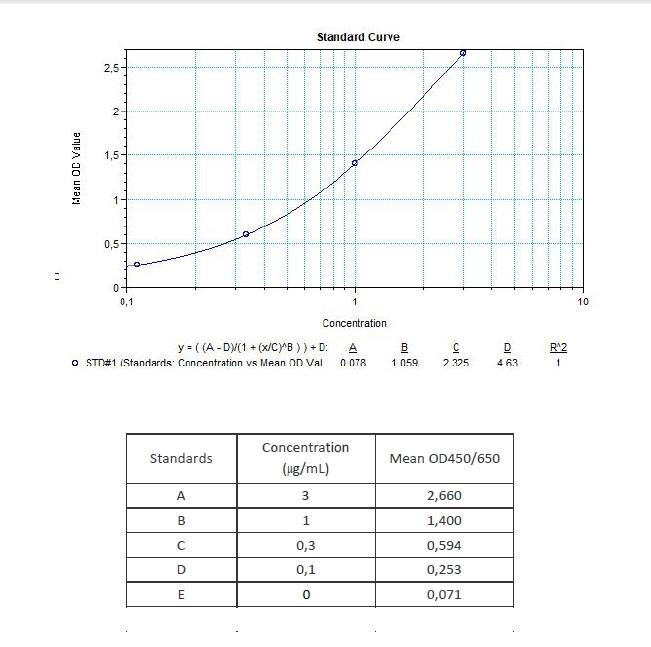
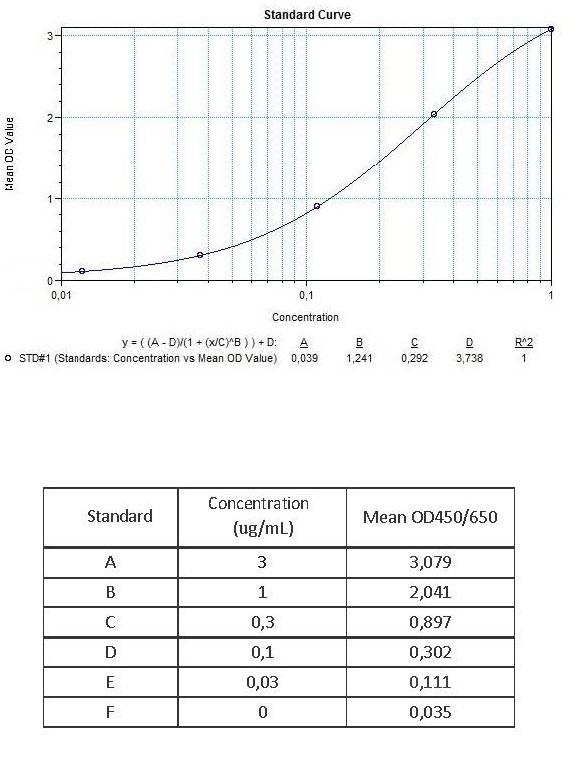
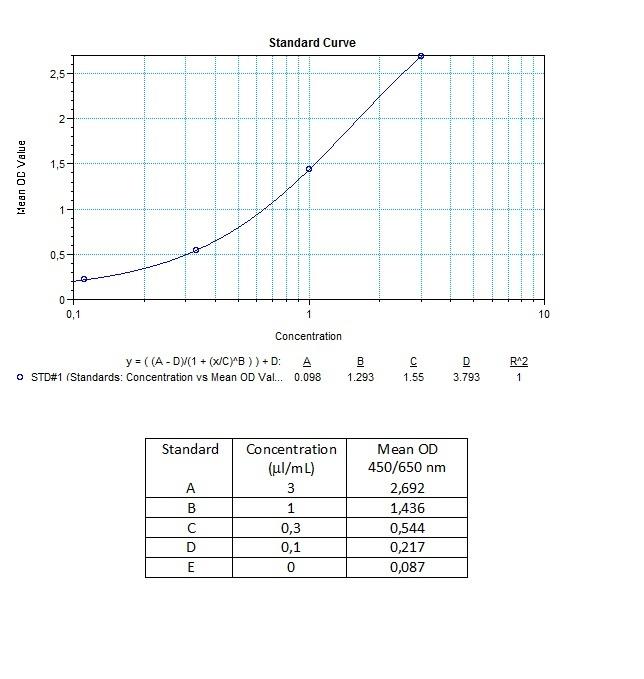
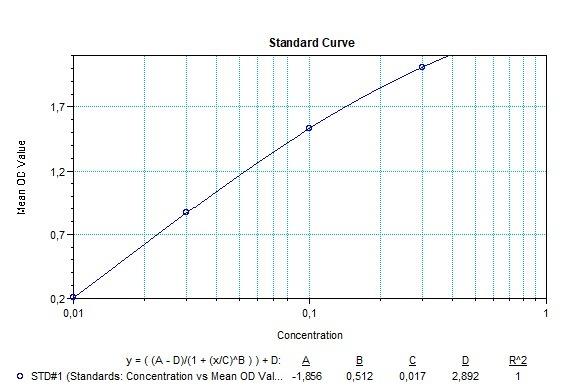
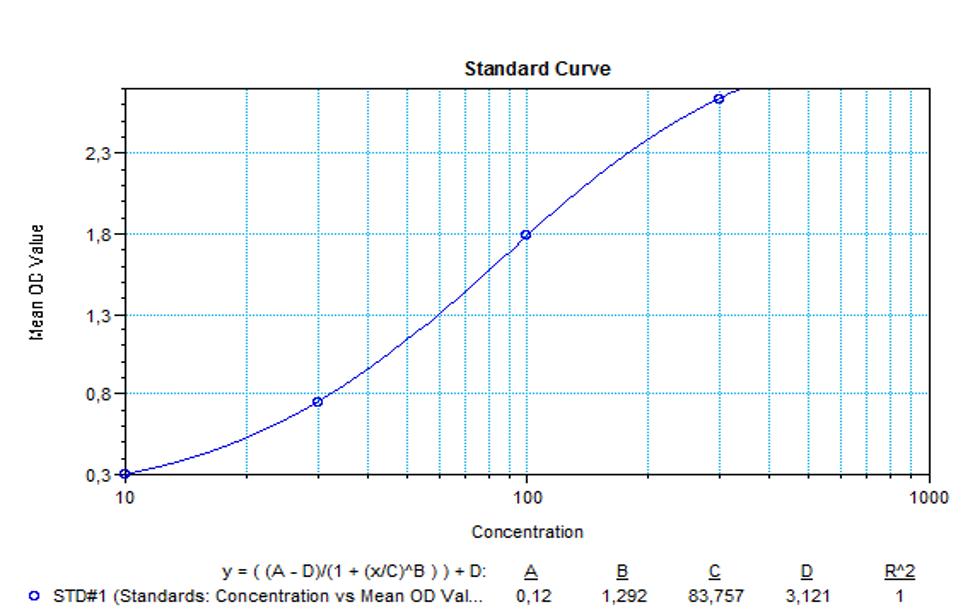
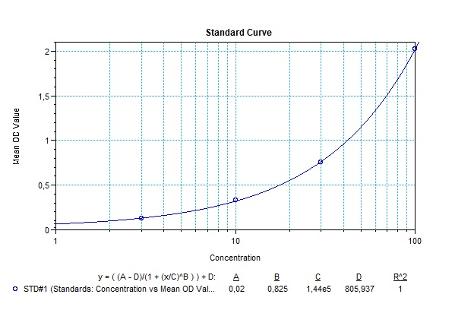
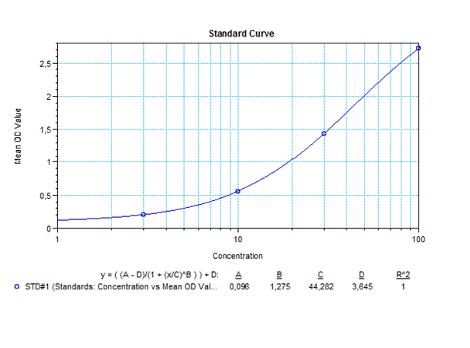
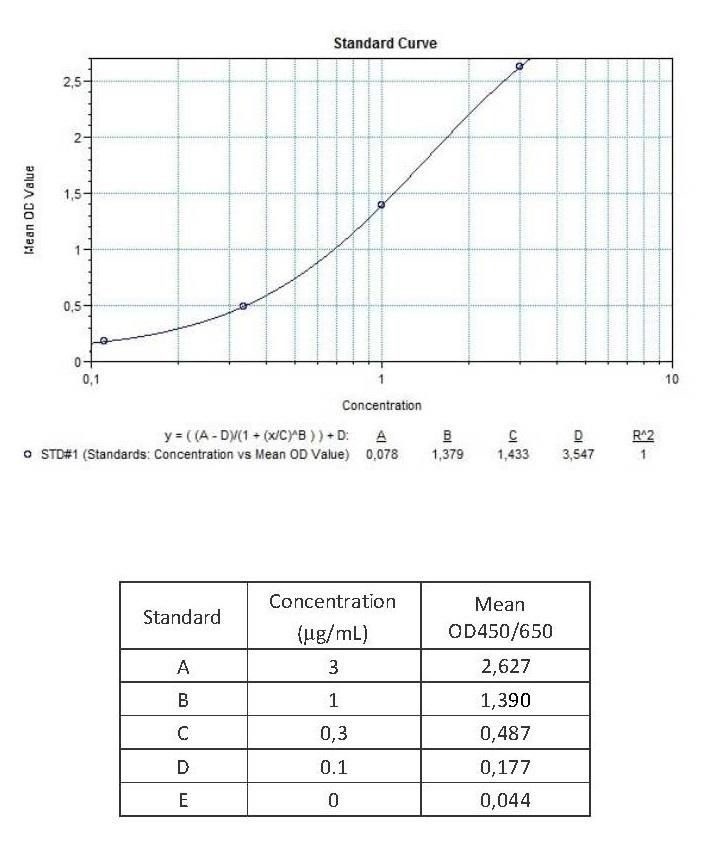
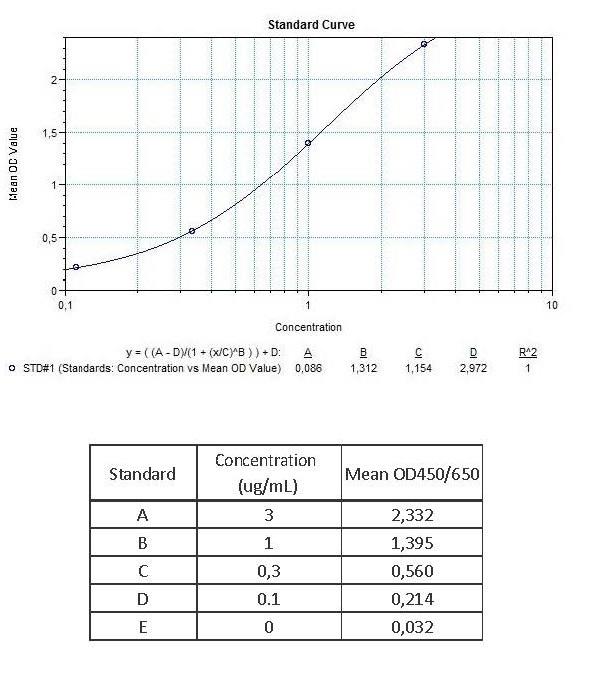
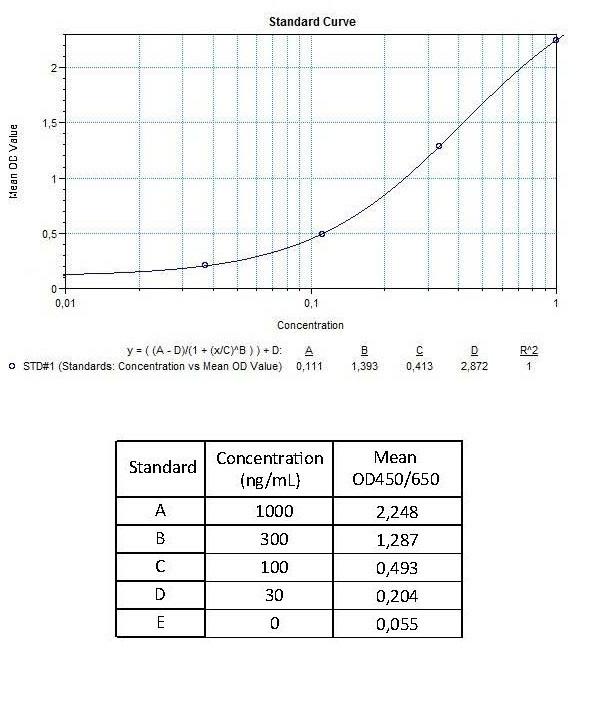
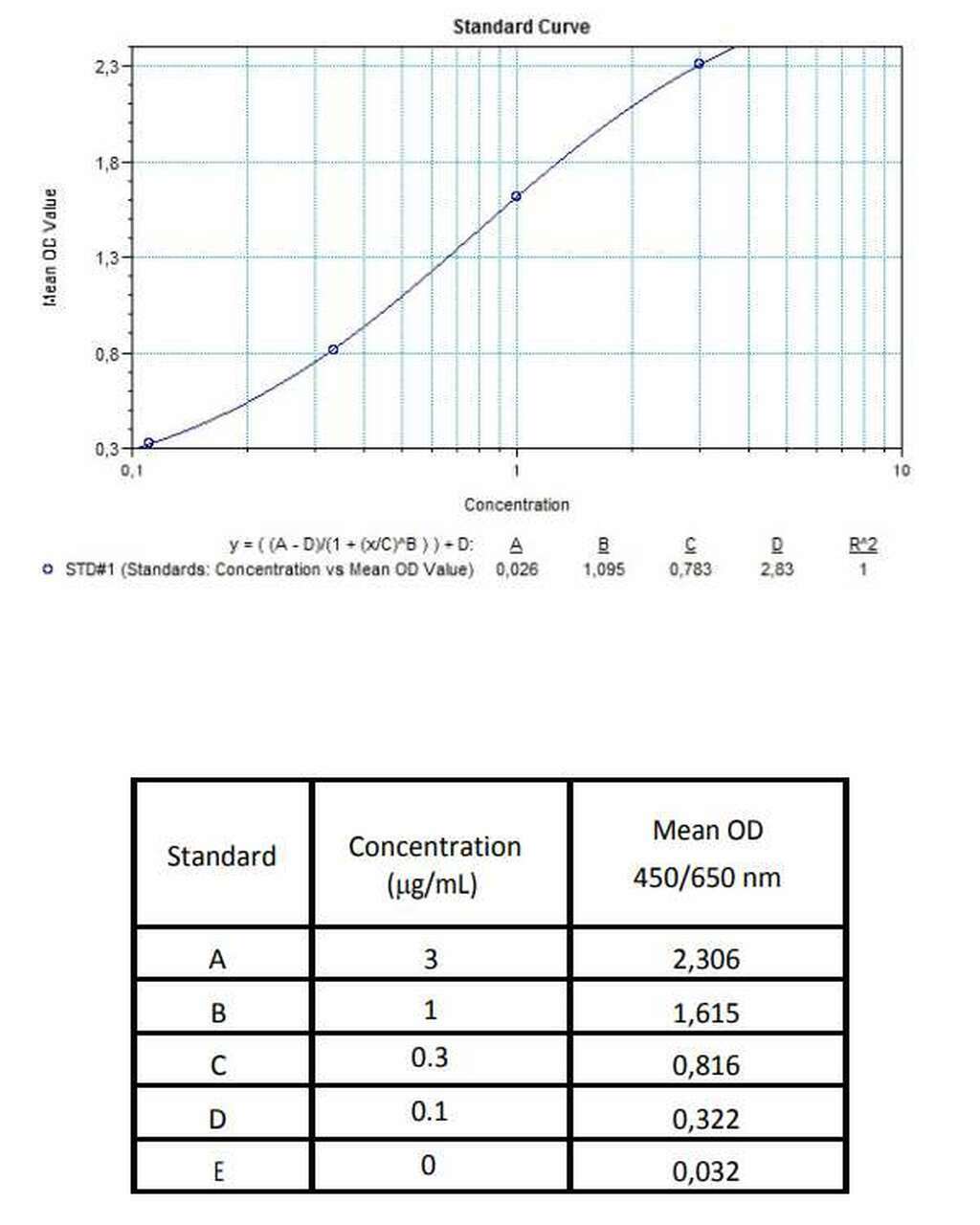
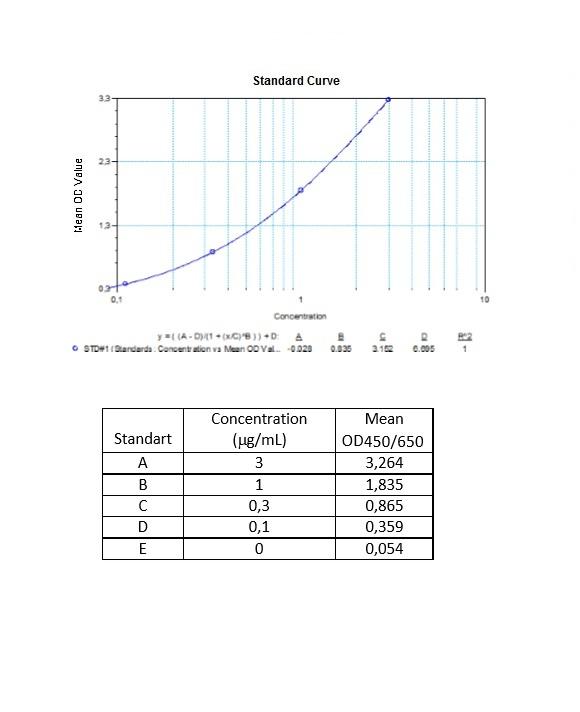
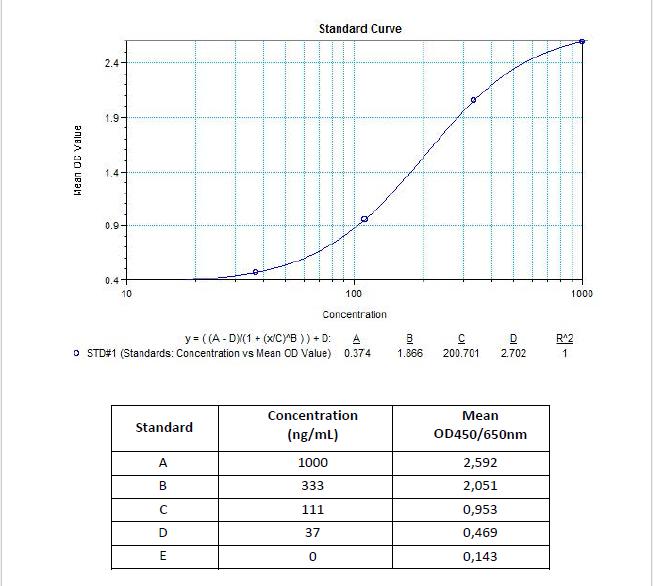
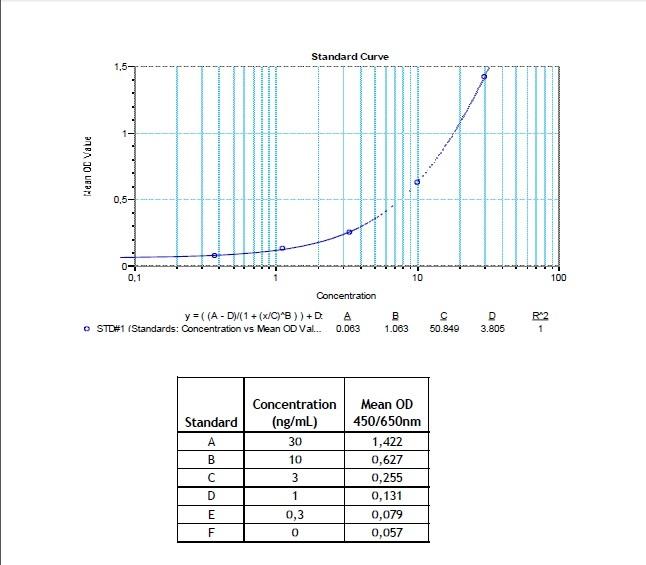
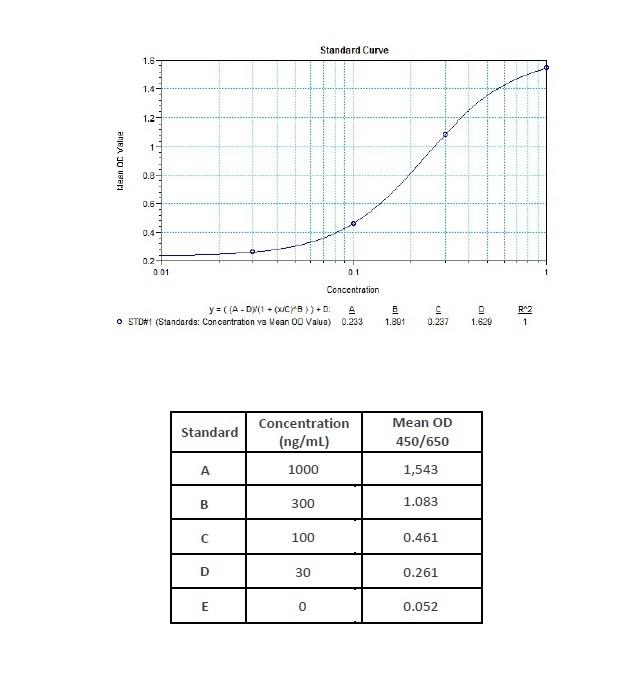
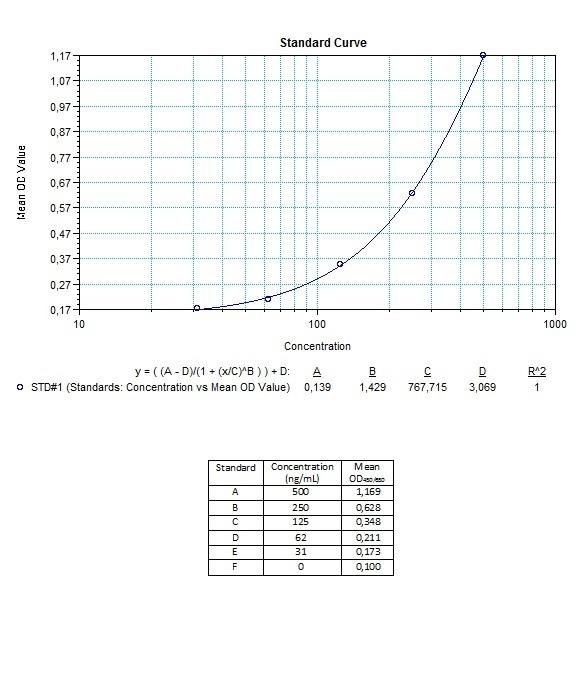
Applications & Data
Therapeutic mAb & Biosimilar ELISA
- Quality Control | ELISA can be used for QC during the development and manufacturing of antibodies
- Biosimilarity assessment | Test similarity between biosimilar and reference product
- PK studies | Assess how mAbs move through the body and help to determine optimal dosing.
- Clinical Development | Monitor the presence of therapeutic & biosimilar mAb in patient samples

Anti-Drug Antibody (ADA) ELISA
- Immunogenicity Assessment | High levels of ADAs may neutralize the therapeutic effects of the drug, rendering it less effective.
- Response Variability | Identify differences in patients response by measuring ADA levels
- Biosimilarity Evaluation | Helps to assess the immunogenicity profile of biosimilars to reference product
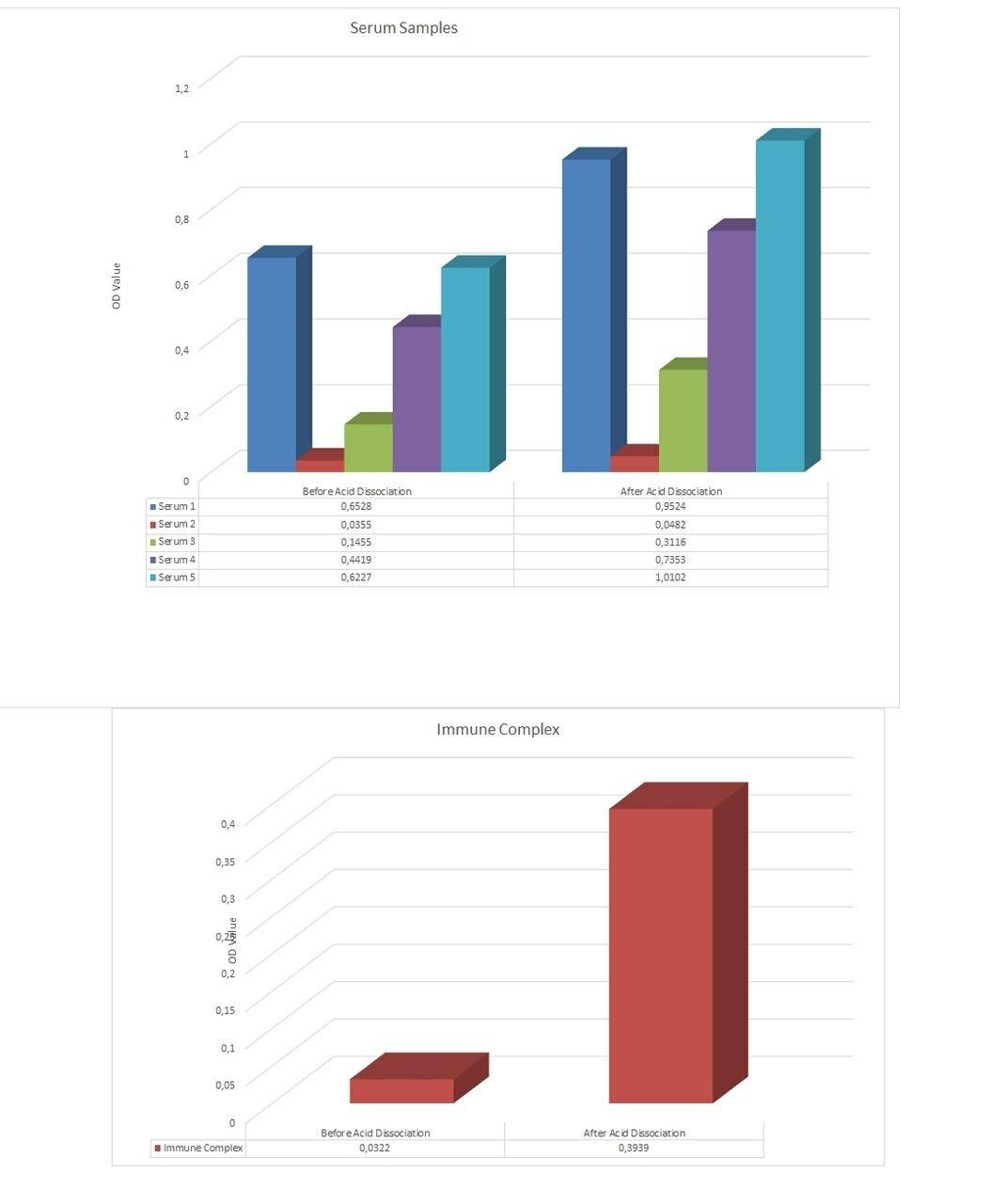
- Free vs. Total ADA | Acid dissociation technology breaks antibody complex making ADA detectable
Meet Some of the Genie Team!
FAQs - Therapeutic mAb & Biosimilar ELISA Kit
1.) How should my ELISA Kit be stored?
2.) There was a weak/no signal in my ELISA results, what could have caused this?
Possible Cause | Possible Solution |
Reagents not at room temperature | All reagents should at room temperature from the start of the assay. Room temperature should be reached following 15–20 minutes on the bench. |
Incubation time too short | Follow manufacturer guidelines in the technical manuals |
Incorrect wavelength | Manufactured kits have optimized protocols. Make sure to use recommended wavelength. Ensure plate reader is set accurately for type of substrate being used |
Target present below detection limits of assay | Decrease dilution factor or concentrate samples |
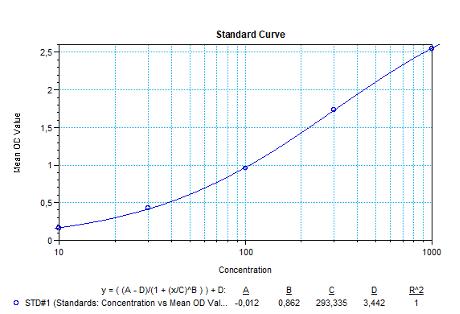
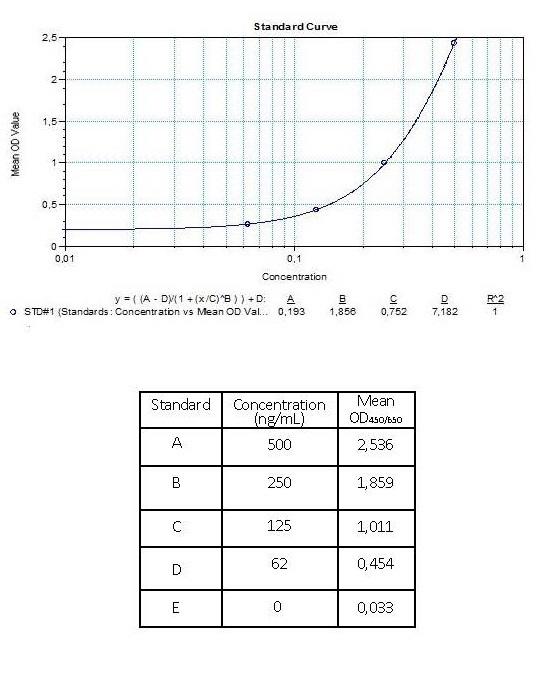
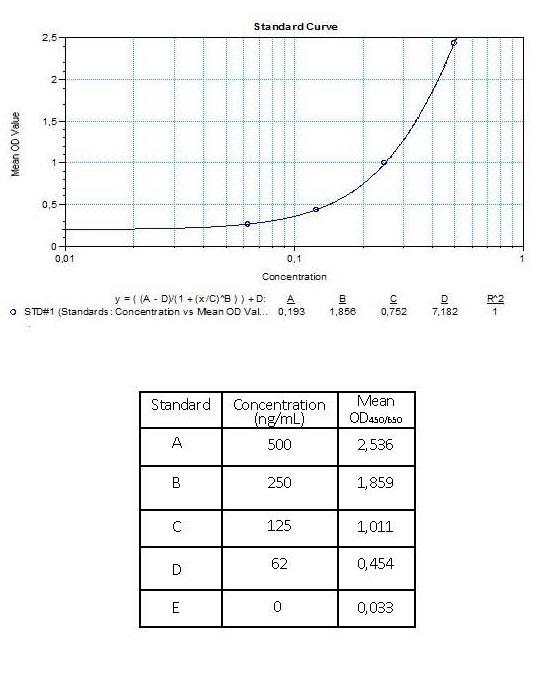
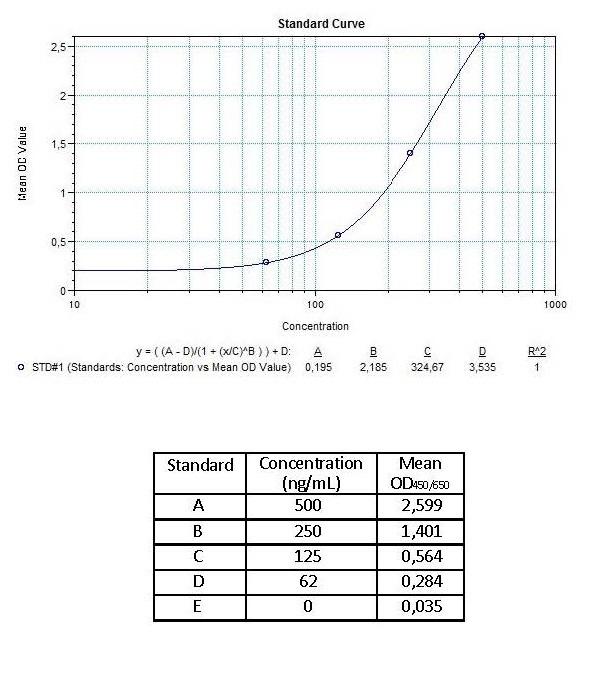
3.) Poor standard curve in results?
Reagents are poorly mixed, the standard has degraded or pipetting errors.
For more troubleshooting suggestions vist our 101 ELISA Troubleshooting Tips!
Research related ELISA Kits
| Anti-Ramucirumab (Cyramza®) ADA | |
|---|---|
| Product Code | HUMB00023 |
| ELISA Type | Antibody screening - Qualitative |
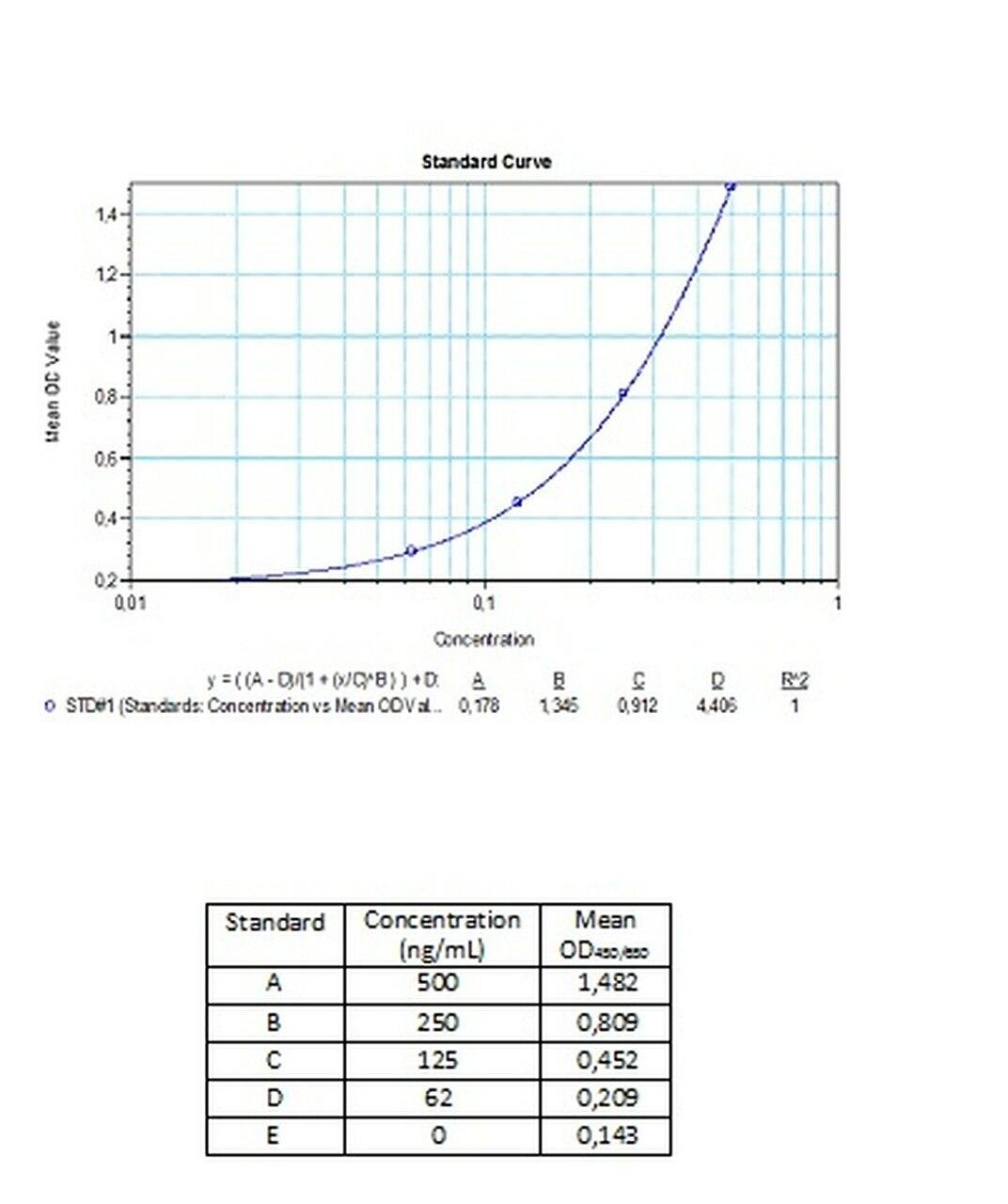
| Anti-Bevacizumab (Avastin®) ADA | |
|---|---|
| Product Code | HUMB00025 |
| ELISA Type | Antibody screening - Qualitative |
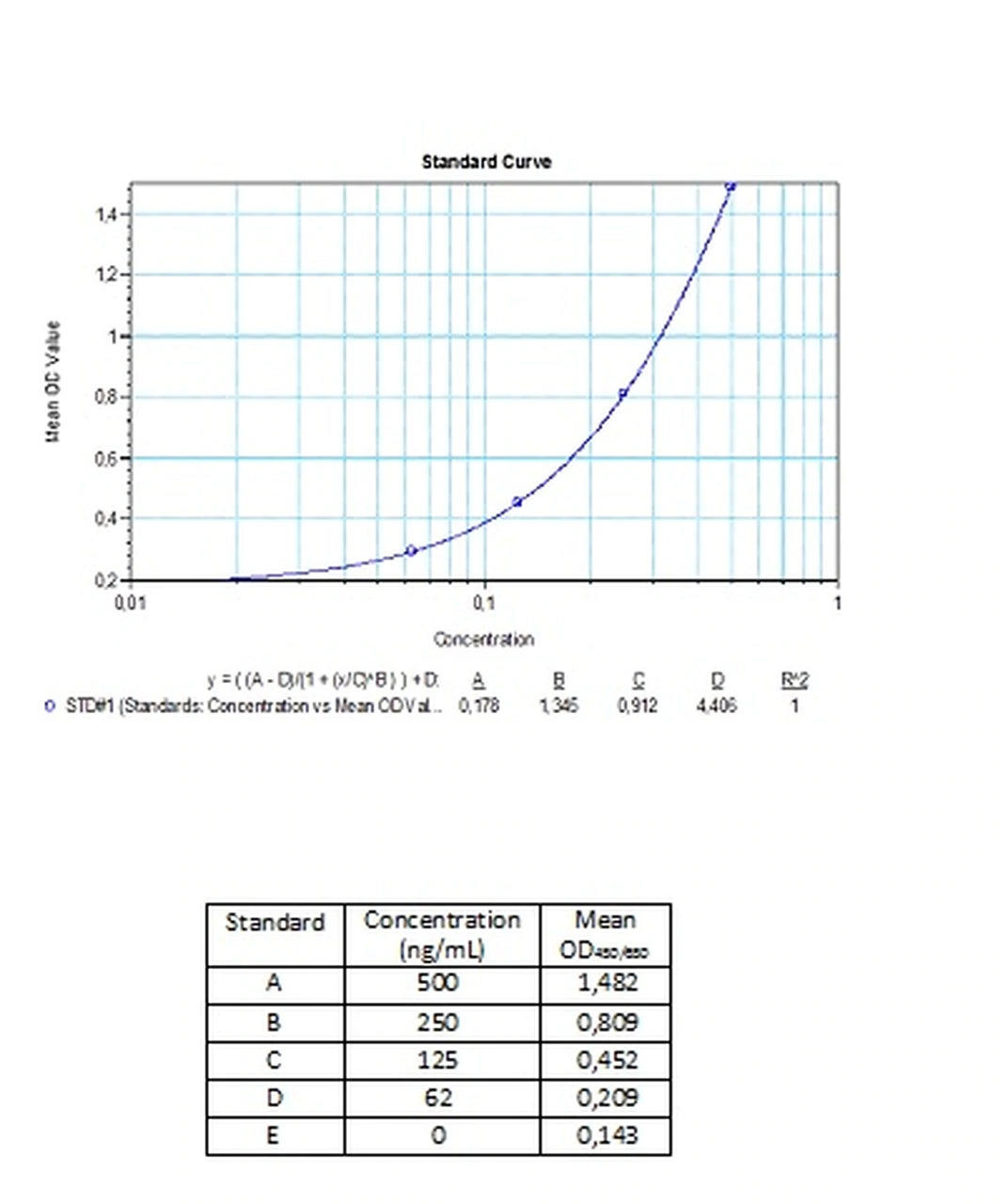
| Anti-Bevacizumab (Avastin®) ADA | |
|---|---|
| Product Code | HUMB00026 |
| ELISA Type | Antibody screening - Quantitative |

| Anti-Rituximab (Rituxan®, Mabthera®) ADA | |
|---|---|
| Product Code | HUMB00025 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Rituximab (Rituxan®, Mabthera®) ADA | |
|---|---|
| Product Code | HUMB00026 |
| ELISA Type | Antibody screening - Quantitative |
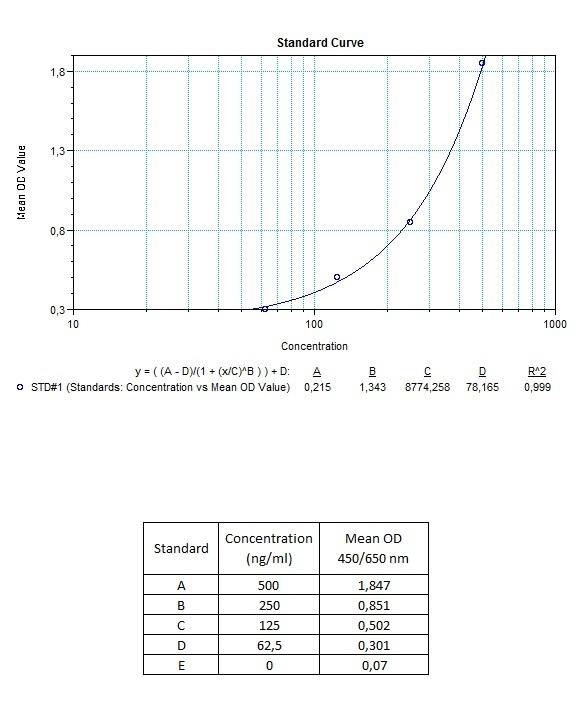
| Anti-Rituximab (Rituxan®, Mabthera®) ADA | |
|---|---|
| Product Code | HUMB00028 |
| ELISA Type | Antibody screening - Qualitative |
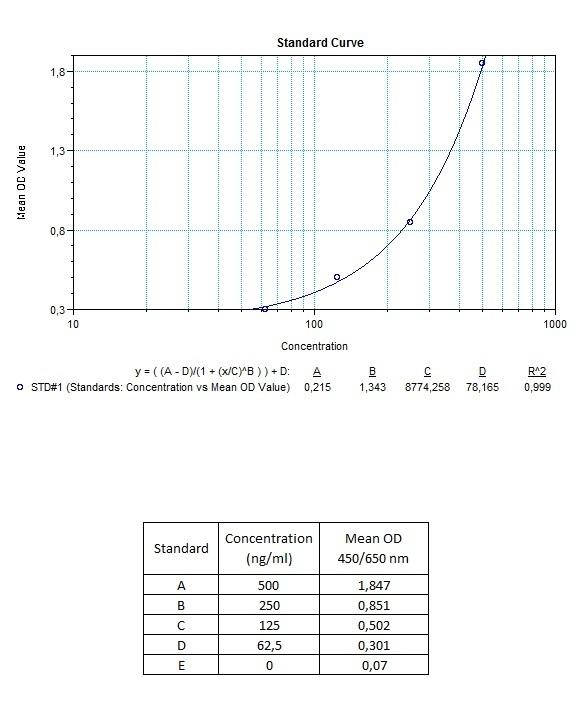
| Anti-Rituximab (Rituxan®, Mabthera®) ADA | |
|---|---|
| Product Code | HUMB00029 |
| ELISA Type | Antibody screening - Quantitative |
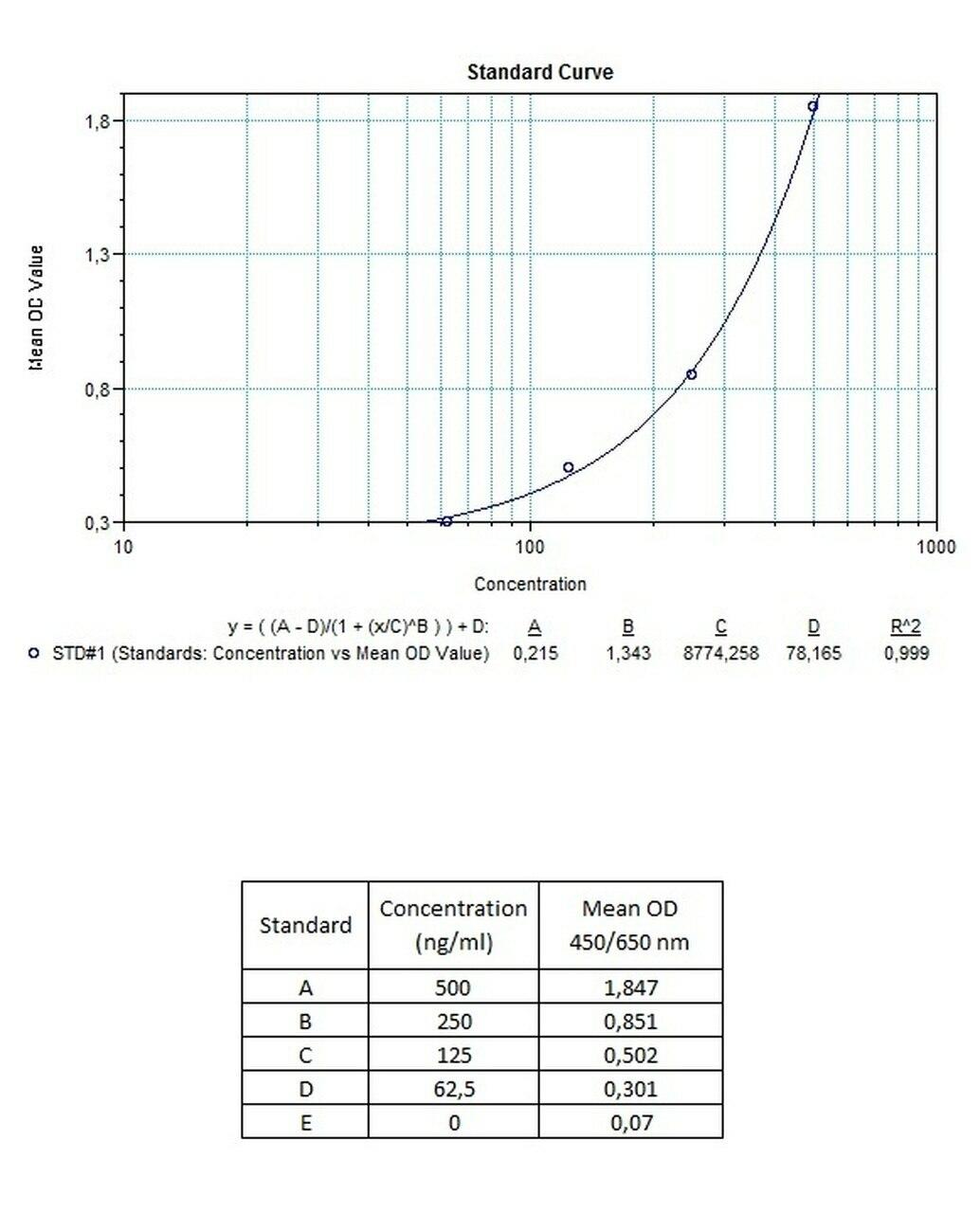
| Anti-Trastuzumab (Herceptin®, Herclon®) ADA | |
|---|---|
| Product Code | HUMB00031 |
| ELISA Type | Antibody screening - Qualitative |
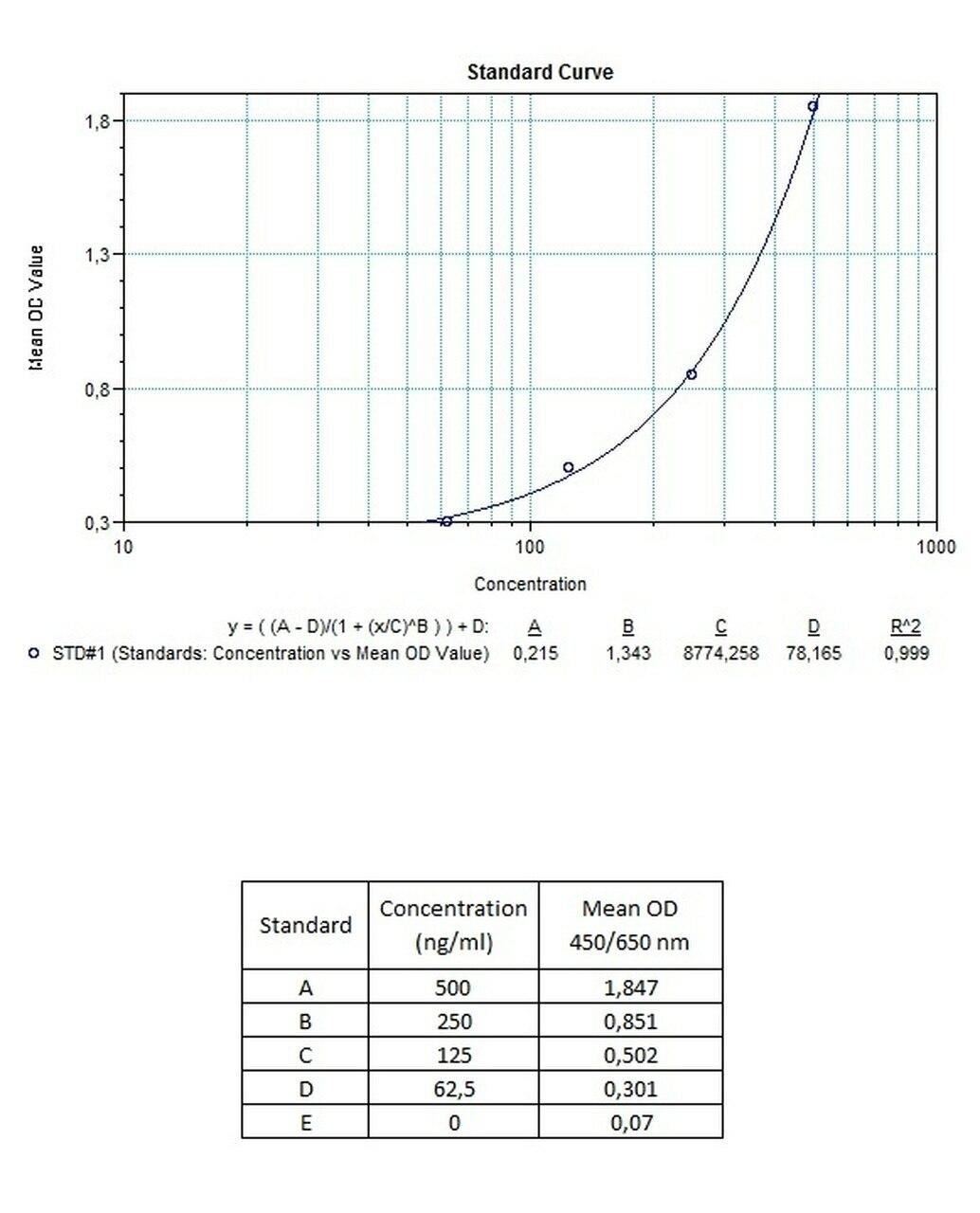
| Anti-Trastuzumab (Herceptin®, Herclon®) ADA | |
|---|---|
| Product Code | HUMB00032 |
| ELISA Type | Antibody screening - Quantitative |
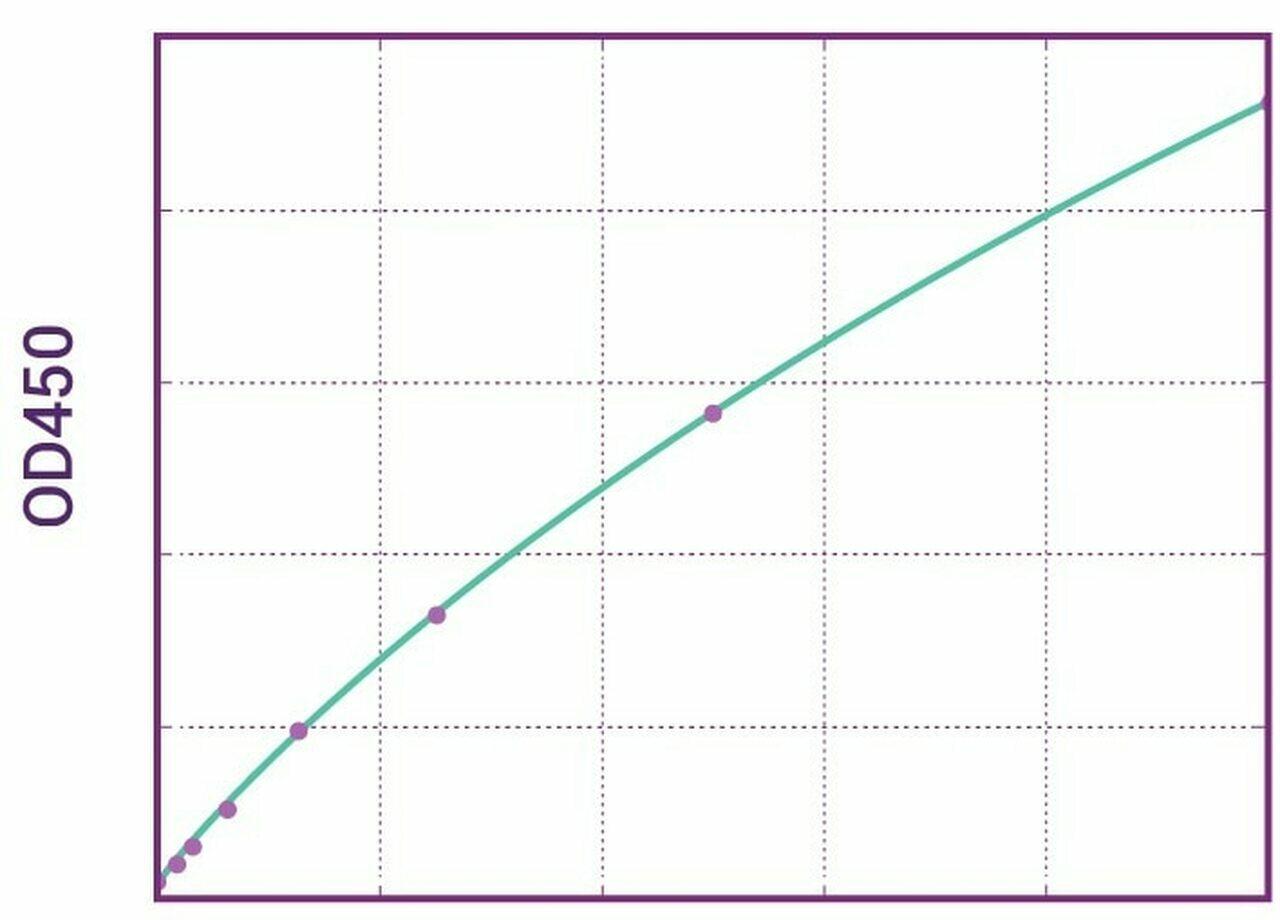
| Anti-Cetuximab (Erbitux®) ADA | |
|---|---|
| Product Code | HUMB00034 |
| ELISA Type | Antibody screening - Qualitative |
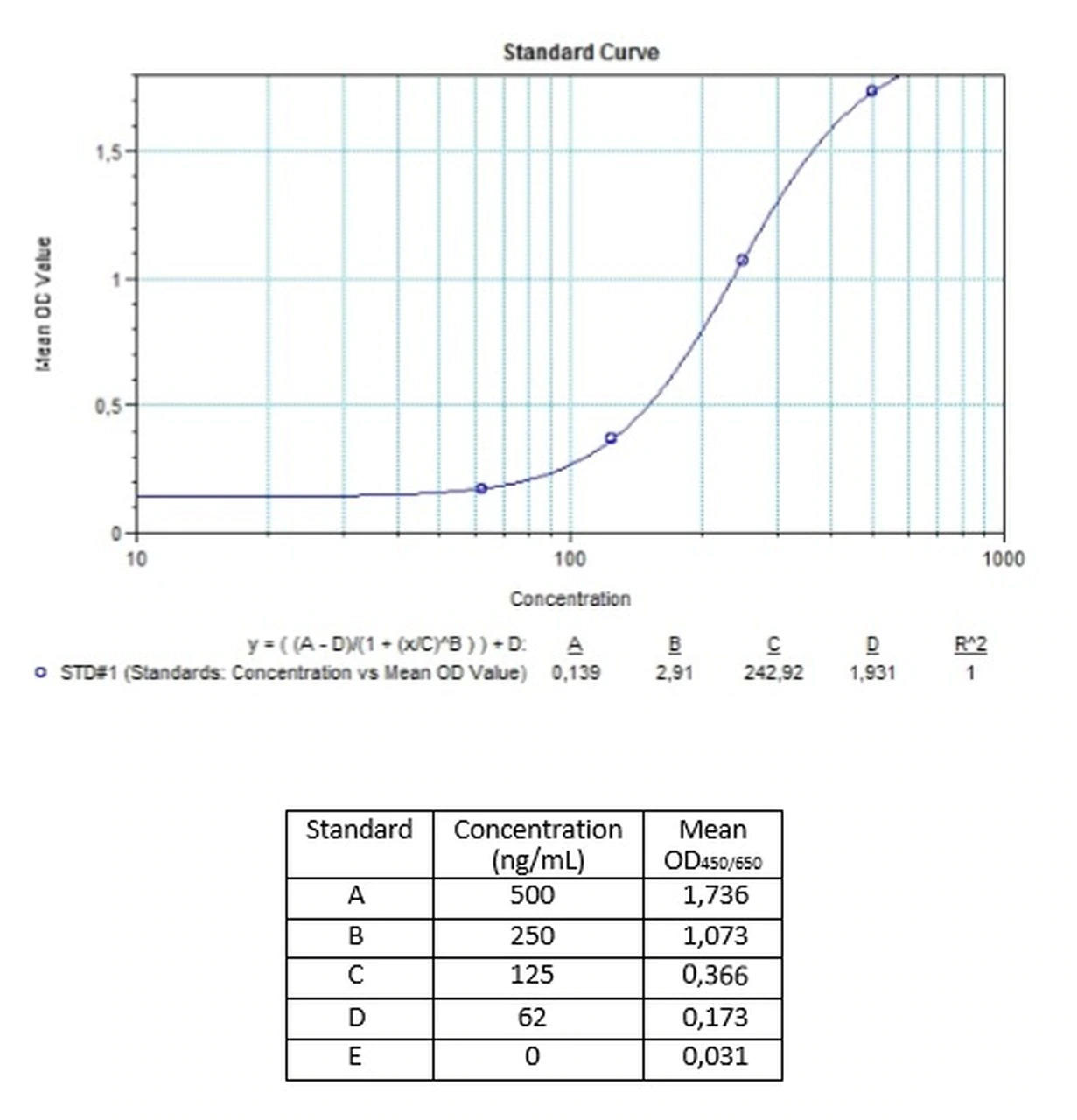
| Anti-Cetuximab (Erbitux®) ADA | |
|---|---|
| Product Code | HUMB00035 |
| ELISA Type | Antibody screening - Quantitative |
| Daratumumab (Darzalex®) ADA | |
|---|---|
| Product Code | HUMB00063 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Avelumab (Bavencio®) ADA | |
|---|---|
| Product Code | HUMB00043 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Nivolumab (Opdivo®) ADA | |
|---|---|
| Product Code | HUMB00045 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Pembrolizumab (Keytruda®) ADA | |
|---|---|
| Product Code | HUMB00047 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Ipilimumab (Yervoy®) ADA | |
|---|---|
| Product Code | HUMB00049 |
| ELISA Type | Antibody screening - Qualitative |
| Atezolizumab (Tecentriq®) ADA | |
|---|---|
| Product Code | HUMB00061 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Vedolizumab (Entyvio®) ADA Qualitative ELISA Kit | |
|---|---|
| Product Code | HUMB00019 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Ustekinumab (Stelara®) ADA | |
|---|---|
| Product Code | HUMB00021 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Tocilizumab (Actemra®) | |
|---|---|
| Product Code | HUMB00051 |
| ELISA Type | Antibody screening - Qualitative |
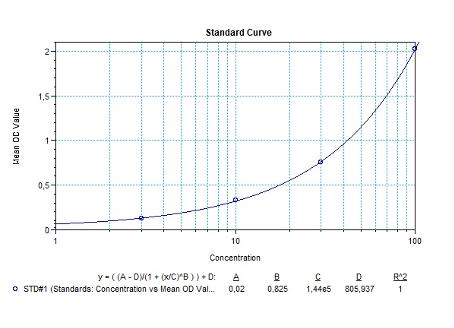
| Anti-Natalizumab (Tysabri®) ADA | |
|---|---|
| Product Code | HUMB00053 |
| ELISA Type | Antibody screening - Qualitative |
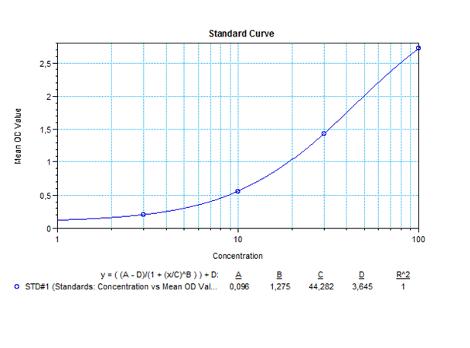
| Anti-Canakinumab (Ilaris®) ADA | |
|---|---|
| Product Code | HUMB00057 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Abatacept (Orencia®) ADA | |
|---|---|
| Product Code | HUMB00069 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Secukinumab (Cosentyx® , Verxant®) ADA | |
|---|---|
| Product Code | HUMB00067 |
| ELISA Type | Antibody screening - Qualitative |
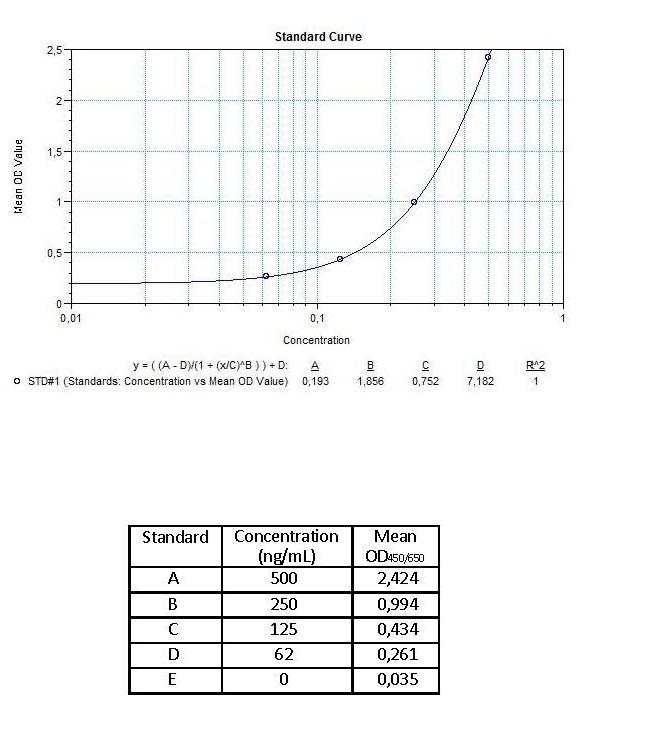
| Anti-Infliximab (Remicade®) ADA | |
|---|---|
| Product Code | HUMB00002 |
| ELISA Type | Antibody screening - Qualitative |
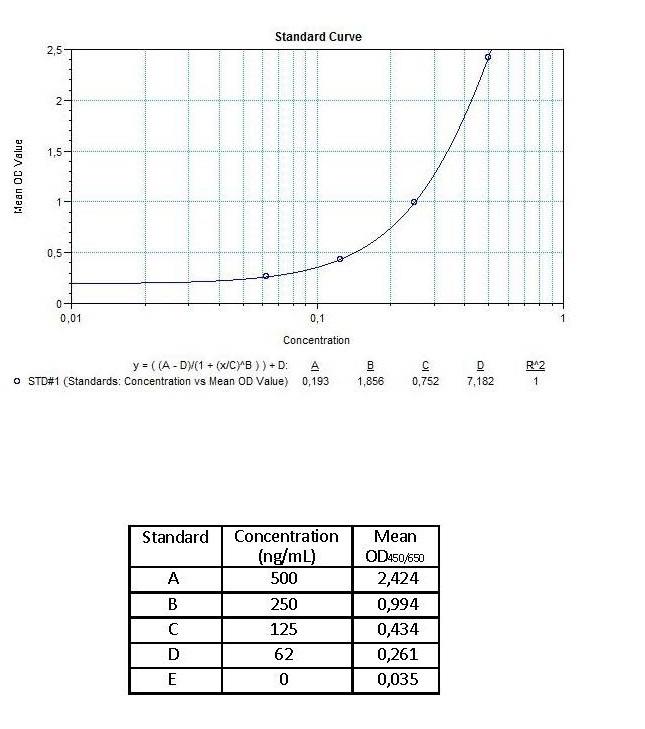
| Anti-Infliximab (Remicade®) ADA | |
|---|---|
| Product Code | HUMB00003 |
| ELISA Type | Antibody screening - Quantitative |
| Anti-Infliximab (Remicade®) Free Drug/ADA Dual ELISA | |
|---|---|
| Product Code | HUMB00004 |
| ELISA Type | Antibody screening - Free/Total semiquantitative |
| Anti-Infliximab (Remsima®) ADA | |
|---|---|
| Product Code | HUMB00006 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Infliximab (Remsima®) ADA | |
|---|---|
| Product Code | HUMB00007 |
| ELISA Type | Antibody screening - Quantitative |

| Anti-Infliximab (Remsima®) ADA | |
|---|---|
| Product Code | HUMB00008 |
| ELISA Type | Antibody screening - Total semiquantitative |
| Anti-Adalimumab (Humira®) ADA | |
|---|---|
| Product Code | HUMB00010 |
| ELISA Type | Antibody screening - Qualitative |
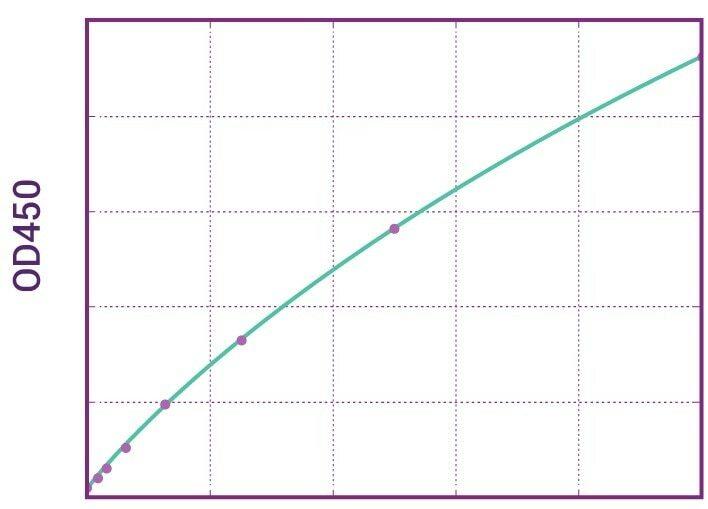
| Anti-Adalimumab (Humira®) ADA | |
|---|---|
| Product Code | HUMB00011 |
| ELISA Type | Antibody screening - Quantitative |
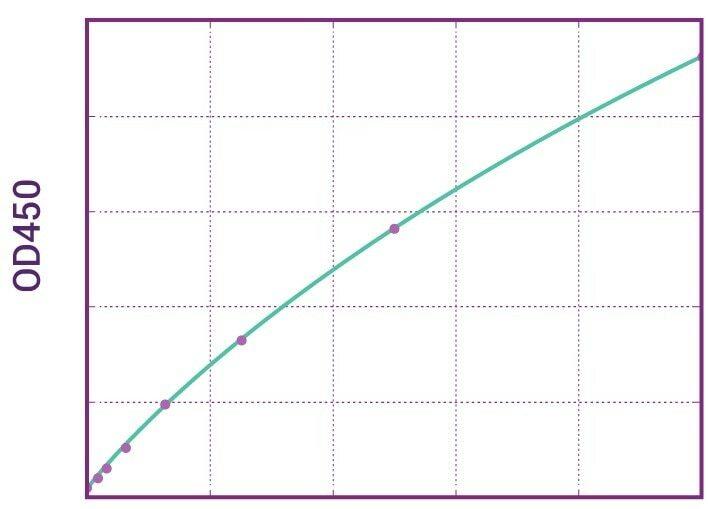
| Anti-Adalimumab (Humira®) Free Drug/ADA Dual ELISA | |
|---|---|
| Product Code | HUMB00012 |
| ELISA Type | Antibody screening - Free/Total semiquantitative |
| Anti-Etanercept (Enbrel®) ADA | |
|---|---|
| Product Code | HUMB00014 |
| ELISA Type | Antibody screening - Qualitative |
| Anti-Golimumab (Simponi®) ADA | |
|---|---|
| Product Code | HUMB00016 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Bevacizumab (Avastin®) ADA | |
|---|---|
| Product Code | HUMB00025 |
| ELISA Type | Antibody screening - Qualitative |

| Anti-Bevacizumab (Avastin®) ADA | |
|---|---|
| Product Code | HUMB00026 |
| ELISA Type | Antibody screening - Quantitative |
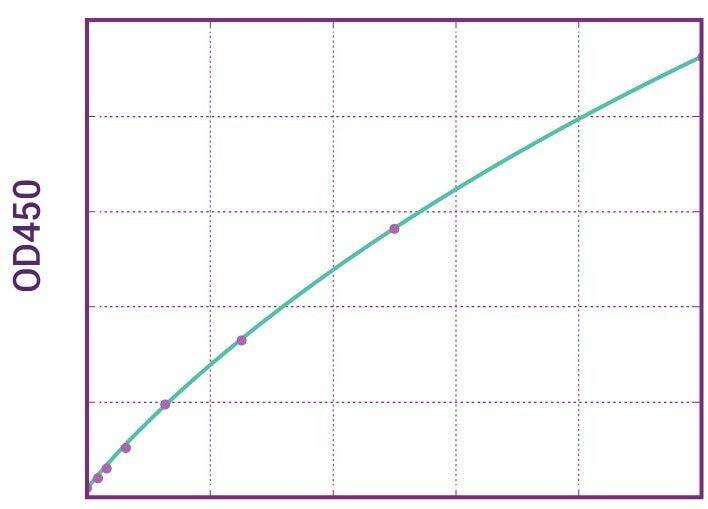
| Anti-Omalizumab (Xolair®) ADA | |
|---|---|
| Product Code | HUMB00040 |
| ELISA Type | Antibody screening - Qualitative |
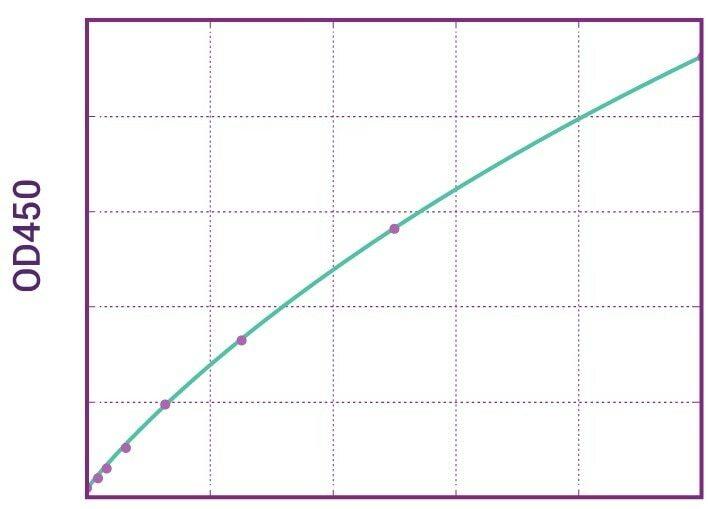
| Anti-Denosumab (Prolia®) ADA | |
|---|---|
| Product Code | HUMB00037 |
| ELISA Type | Antibody screening - Qualitative |