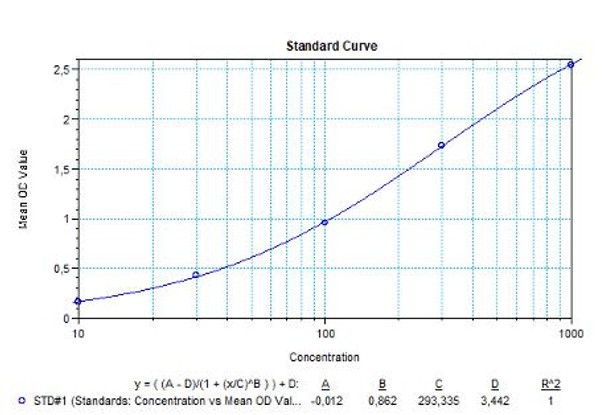
Daratumumab (Darzalex®)Free drug ELISA Kit
- SKU:
- HUMB00062
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Free drug
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Daratumumab (Darzalex®)
- Research Area:
- Anti-Cancer
Description
Daratumumab (Darzalex®) Free drug ELISA Kit
Assay Genie Daratumumab ELISA has been especially developed for the quantitative analysis of free Daratumumab in serum and plasma samples. Assay Genie Daratumumab ELISA is optimized with Darzalex®.
Daratumumab (Darzalex®) Free drug ELISA Kit Test Principle
Solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich principle. Standards and samples (serum or plasma) are incubated in the microtiter plate coated with the reactant for daratumumab. After incubation, the wells are washed. Then, horse radish peroxidase (HRP) conjugated probe is added and binds to daratumumab captured by the reactant on the surface of the wells. Following incubation wells are washed and the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen substrate. Finally, the reaction is terminated with an acidic stop solution. The colour developed is proportional to the amount of daratumumab in the sample or standard. Results of samples can be determined directly using the standard curve.
Daratumumab (Darzalex®) Free drug ELISA Kit Product Information
| Information | Description |
| Application | Free Drug |
| Required Volume | 10 µL |
| Total Time | 70 Minutes |
| Sample Type | Serum, Plasma |
| Number of Assays | 96 |
| Detection Limit | 30 ng/mL |
| Spike Recovery | 85-115% |
| Shelf Life (year) | 1 |
| Alternative Names | - |
About Daratumumab (Darzalex®) Free drug ELISA Kit
Daratumumab is indicated as an intravenous injection alone or in combination with other medications for the treatment of multiple myeloma. Subcutaneous daratumumab with hyaluronidase is also indicated alone or in combination for the treatment of multiple myeloma.
Daratumumab is a monoclonal antibody that targets and induces apoptosis in cells that highly express CD38, including multiple myeloma cells. It has a long duration of action as it is given every 1-4 weeks. Patients should be counselled regarding the risk of hypersensitivity, neutropenia, thrombocytopenia, embryo-fetal toxicity, and interferences with cross-matching and red blood cell antibody screening.
CD38 is a glycoprotein present on the surface of hematopoietic cells and is responsible for a number of cell signalling functions. Daratumumab is an immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that targets CD38. Cancers like multiple myeloma overexpress CD38, allowing daratumumab to have higher affinity for these cells. This binding allows daratumumab to induce apoptosis, antibody dependent cellular phagocytosis, and antibody and complement-dependent cytotoxicity. Antibody dependent cellular phagocytosis is mediated by the FC region of the antibody inducing phagocytes 3 such as macrophages, antibody dependent cellular cytotoxicity is mediated by the FC region of the antibody inducing effector cells such as natural killer cells, and complement dependent cytotoxicity is mediated by the FC region of the antibody binding to and inducing complement protein activity.