CD86: Enhancing T Cell Activation in Immunotherapy
Introduction to CD86 and Its Role in Immune Activation
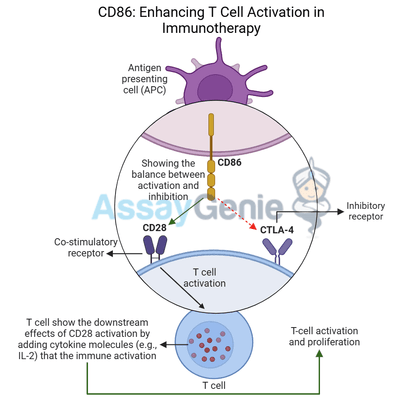
CD86 is a crucial co-stimulatory molecule that plays an essential role in T cell activation, driving the immune system’s ability to recognize and destroy infected or cancerous cells. Expressed primarily on antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells, CD86 binds to receptors on T cells to regulate their activity. CD86 interacts with CD28 to provide a critical co-stimulatory signal necessary for full T cell activation, promoting proliferation, cytokine production, and effector functions in immune responses.
In the context of cancer immunotherapy, harnessing CD86's co-stimulatory functions is pivotal in enhancing anti-tumor immunity. By engaging with CD28, CD86 ensures that T cells receive the secondary signals required to fully activate and eliminate cancer cells. However, CD86 can also bind to CTLA-4, an inhibitory receptor on T cells that dampens immune responses. This dual role in both immune activation and inhibition makes CD86 an attractive target in cancer immunotherapy, as modulating its activity can enhance T cell responses against tumors while preventing immune suppression.
Monoclonal antibodies like GL1, which target CD86, are being developed to boost immune responses in cancer treatment. This article explores the biology of CD86, its function in T cell activation, and the potential of CD86-targeting therapies like GL1 in cancer immunotherapy.
The Structure and Function of CD86 in T Cell Activation
CD86 and Its Interaction with CD28
CD86 belongs to the B7 family of co-stimulatory molecules that regulate T cell activity. When antigen-presenting cells (APCs) present antigens to T cells via the T cell receptor (TCR), the engagement of CD86 with CD28 provides the second signal—known as co-stimulation—that is required for full T cell activation. This co-stimulatory signal is essential for:
- T cell proliferation: CD28 signaling enhances the expansion of activated T cells, ensuring a
robust immune response. - Cytokine production: Co-stimulation by CD86 increases the production of IL-2, a key cytokine that drives T cell growth and function.
- Survival of T cells: CD86 engagement with CD28 promotes the survival and differentiation of T cells into effector T cells, which are crucial for targeting and eliminating tumor cells.
Through this interaction with CD28, CD86 amplifies T cell responses, allowing the immune system to mount an effective attack against cancerous or infected cells.
CD86 and Its Interaction with CTLA-4
In addition to providing co-stimulatory signals through CD28, CD86 can also bind to CTLA-4, a checkpoint receptor expressed on activated T cells and regulatory T cells (Tregs). Unlike CD28, CTLA-4 delivers inhibitory signals that suppress T cell activity and promote immune tolerance. The interaction between CD86 and CTLA-4 results in:
- Inhibition of T cell proliferation: CTLA-4 signaling reduces the expansion of activated T cells, limiting the immune response.
- Promotion of immune tolerance: CTLA-4 engagement enhances the function of Tregs, which suppress effector T cells and help maintain immune balance.
This ability of CD86 to either activate or inhibit T cells makes it a critical checkpoint in regulating immune responses, particularly in cancer, where tumors can exploit CTLA-4 to suppress immune activity and promote immune evasion.
CD86 in Cancer Immunotherapy
CD86 and Immune Evasion in the Tumor Microenvironment
In the tumor microenvironment (TME), cancer cells and tumor-associated immune cells often manipulate checkpoint pathways to suppress the immune response and evade destruction. Tumors may upregulate CTLA-4 ligands like CD86 to suppress T cell activity and promote immune evasion. By engaging CTLA-4 on T cells, CD86 contributes to creating an immunosuppressive environment that allows tumors to grow unchecked.
High levels of CD86 expression on tumor-associated macrophages (TAMs) and dendritic cells are frequently observed in cancers such as:
In these cancers, the CD86-CTLA-4 interaction helps to suppress the immune response, reducing the ability of cytotoxic T cells (CTLs) to infiltrate the tumor and eliminate cancer cells.
Therapeutic Potential of Targeting CD86 in Cancer
Targeting CD86 in cancer immunotherapy aims to block its inhibitory interactions with CTLA-4 while preserving or enhancing its co-stimulatory effects through CD28. Monoclonal antibodies like GL1 are designed to disrupt the CD86-CTLA-4 pathway, tipping the balance toward immune activation by promoting T cell responses and reducing immunosuppression.
Therapies targeting CD86 offer several potential benefits in cancer treatment:
- Restoring T cell activity: Blocking the interaction between CD86 and CTLA-4 prevents inhibitory signals from suppressing T cell activity, allowing for stronger immune responses against tumors.
- Reducing Treg-mediated suppression: Targeting CD86 can reduce the activity of regulatory T cells (Tregs), which suppress effector T cells and contribute to immune evasion in the TME.
- Enhancing checkpoint inhibitor efficacy: CD86-targeting therapies can be used in combination with checkpoint inhibitors like anti-PD-1 and anti-CTLA-4 to boost immune activation and improve treatment outcomes.
GL1: A Monoclonal Antibody Targeting CD86
Mechanism of Action of GL1
GL1 is a monoclonal antibody that targets CD86, preventing its interaction with CTLA-4 and promoting its co-stimulatory engagement with CD28. The mechanism of action of GL1 involves:
- Blocking CD86-CTLA-4 interactions: By binding to CD86, GL1 prevents it from delivering inhibitory signals through CTLA-4, thereby reducing immune suppression.
- Promoting CD86-CD28 co-stimulation: GL1 enhances the ability of CD86 to bind to CD28, amplifying T cell activation and proliferation.
- Boosting T cell-mediated cytotoxicity: Increased co-stimulation via CD86-CD28 interactions results in greater cytokine production and effector T cell activity, promoting the destruction of cancer cells.
- Modulating the tumor microenvironment: GL1 can shift the TME toward a more immune-activating state, reducing the suppressive influence of Tregs and enhancing the infiltration of cytotoxic T cells.
Clinical Applications of GL1
GL1 has potential therapeutic applications in a range of cancers where CD86-mediated immunosuppression plays a significant role in tumor progression. These include:
- Melanoma: CD86 is upregulated in the melanoma tumor microenvironment, and blocking
CD86-CTLA-4 interactions can restore T cell activity. - NSCLC: Non-small cell lung cancer is often associated with high levels of immunosuppressive TAMs expressing CD86. GL1 could enhance T cell responses and improve the efficacy of checkpoint inhibitors.
- Colorectal cancer: CD86 contributes to immune evasion in colorectal cancer, and targeting
CD86 with GL1 can help overcome this resistance and promote stronger anti-tumor immunity.
Cancer Type | CD86 Expression | Therapeutic Potential of CD86 Blockade (GL1) |
|---|---|---|
High expression in the tumor microenvironment | Blocking CD86-CTLA-4 interactions restores T cell activity. | |
Elevated CD86 on TAMs and dendritic cells | GL1 enhances T cell responses and boosts checkpoint inhibitor efficacy. | |
CD86 contributes to immune evasion | Targeting CD86 reduces Treg-mediated suppression and enhances immune activation. |
Synergy with Checkpoint Inhibitors and Other Therapies
Combination with Anti-PD-1 and Anti-CTLA-4 Therapies
Checkpoint inhibitors such as anti-PD-1 and anti-CTLA-4 have transformed cancer treatment by unleashing the immune system’s ability to attack tumors. However, some cancers remain resistant to these therapies due to the presence of multiple immune-suppressive pathways. Targeting CD86 with GL1 can complement these treatments by:
- Enhancing T cell activation: GL1 promotes CD86-CD28 co-stimulation, making T cells more responsive to checkpoint inhibition.
- Overcoming immunotherapy resistance: In tumors where PD-1 or CTLA-4 blockade alone is
insufficient, CD86-targeting therapies provide an additional mechanism for enhancing immune responses.
Potential for Combining CD86 Blockade with Chemotherapy
Chemotherapy can trigger the release of tumor antigens, which are recognized by the immune system. However, chemotherapy often induces immunosuppression that limits the effectiveness of the anti-tumor immune response. Targeting CD86 with GL1 could enhance the immune system’s ability to recognize and respond to these antigens, making chemotherapy more effective.
Challenges and Future Directions in CD86-Targeted Therapy
Managing Immune-Related Toxicities
Targeting CD86 to boost immune responses carries the risk of immune-related adverse events (irAEs), including autoimmune reactions and inflammation. Given the dual role of CD86 in both immune activation and inhibition, careful dosing and patient selection will be critical to balancing efficacy with safety.
Expanding the Use of CD86 Blockade
While early research into CD86-targeting therapies is promising, future studies will likely explore the application of these therapies in a broader range of cancers. Identifying biomarkers that predict patient response to CD86 blockade will be crucial for maximizing the effectiveness of these therapies.
Conclusion
CD86 is a key immune checkpoint molecule that plays a pivotal role in regulating T cell activation through co-stimulatory pathways. By targeting CD86 with therapies like GL1, cancer immunotherapy can amplify T cell responses, reduce immune suppression, and enhance the effectiveness of existing treatments such as checkpoint inhibitors. As research into CD86-targeting therapies continues, these strategies hold great potential for improving outcomes in immune-resistant cancers and expanding the therapeutic toolkit in the fight against cancer.
References
- Greenwald, R.J., Freeman, G.J., Sharpe, A.H., 2005. The B7 family revisited. Annual Review of Immunology, 23, pp.515-548.
- Linsley, P.S., et al., 1991. Binding of the B7 family of proteins by the CD28 receptor on T cells mediates co-stimulation. The Journal of Experimental Medicine, 174(3), pp.561-569.
- Chambers, C.A., et al., 1997. The CD28-B7 pathway in the regulation of T-cell responses. Current Opinion in Immunology, 9(3), pp.396-404.
- Quezada, S.A., Peggs, K.S., Simpson, T.R., et al., 2011. CTLA4 blockade and Treg depletion cooperate to enhance T cell responses in vivo. The Journal of Experimental Medicine, 208(7),
pp.1491-1503. - Walunas, T.L., Lenschow, D.J., Bakker, C.Y., et al., 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity, 1(5), pp.405-413.
- Chambers, C.A., et al., 1999. The CD28-B7 pathway in the regulation of T-cell responses. Immunological Reviews, 169(1), pp.55-66.
- Schneider, H., et al., 2002. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. Journal of Immunology, 169(6), pp.3048-3054.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025