CD122: Fine-Tuning T Cell Responses in Immunotherapy
Introduction to CD122 in Immunotherapy
CD122, also known as the interleukin-2 receptor beta chain (IL-2Rβ), is a critical component of the immune system's response to pathogens and malignancies. Its primary function is to mediate signaling through the IL-2 and IL-15 cytokine pathways, which are essential for T cell proliferation, survival, and differentiation. These T cells, particularly CD8+ T cells and natural killer (NK) cells, play a central role in the body’s immune defense against cancer and infections.
In recent years, immunotherapy has become a transformative approach in cancer treatment. The ability to harness and modify the body's own immune system to fight tumors has opened new therapeutic avenues. CD122 has emerged as a significant target for these therapies, especially in T cell-based treatments, where modulating CD122 can amplify the immune response. This article explores the role of CD122 in T cell regulation, its potential as a therapeutic target, and the promising outcomes from using agents like TM-β1, a monoclonal antibody that targets this receptor.
Structure and Function of CD122 in T Cell Signaling
CD122 forms part of a complex receptor system that includes two other key components: IL-2Rα (CD25) and γc (CD132). Together, these three subunits assemble to form a high-affinity receptor for IL-2 and IL-15, essential cytokines for immune regulation. CD122's role is not only to bind these cytokines but also to transmit crucial intracellular signals that dictate T cell behavior.
The CD122-Containing IL-2 Receptor Complex
Receptor Subunit | Function |
|---|---|
Binds IL-2 with low affinity, facilitates formation of the full receptor. | |
Central to signal transduction, binds IL-2 and IL-15 with intermediate affinity. | |
Essential for signal transduction, shared by other cytokines like IL-7 and IL-21. |
IL-2 and IL-15 Signaling Pathways
- IL-2 signaling: Critical for T cell proliferation and differentiation, particularly in CD8+ T cells. Upon binding to the IL-2R, a cascade is initiated that activates pathways such as JAK/STAT, PI3K/AKT, and MAPK, which are key to T cell expansion and survival.
- IL-15 signaling: Although similar to IL-2 in promoting T cell survival, IL-15 plays a more specialized role in the maintenance of memory T cells and the activation of NK cells. By engaging CD122, IL-15 enhances long-lived CD8+ memory T cells, which are critical for sustained immune responses in both infection and cancer.
These signaling pathways have broad implications for immunotherapy, as they offer mechanisms to either amplify or suppress immune responses based on therapeutic need.
CD122 in T Cell-Mediated Immune Responses
Role of CD122 in CD8+ T Cells
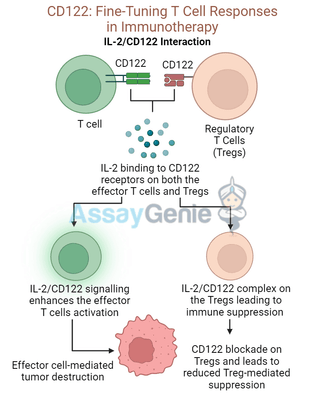
CD8+ T cells are the primary effector cells in the immune system’s fight against cancer. The engagement of CD122 through IL-2 or IL-15 stimulates these cells to proliferate, differentiate into cytotoxic T lymphocytes (CTLs), and target tumor cells. This makes CD122 signaling crucial in the context of cancer immunotherapy.
Research has shown that enhancing IL-2 signaling by targeting CD122 can lead to a significant increase in T cell-mediated tumor destruction. However, because IL-2 also promotes the expansion of regulatory T cells (Tregs), which suppress immune responses, there is a delicate balance to maintain. Selective modulation of CD122 could skew the response toward CD8+ effector T cells, optimizing anti-tumor activity.
Regulatory T Cells and Autoimmunity
Regulatory T cells (Tregs) express high levels of CD25 and also rely on IL-2 signaling for their function. These cells are essential for maintaining immune tolerance and preventing autoimmunity. However, in autoimmune diseases such as rheumatoid arthritis or multiple sclerosis, excessive Treg activity can contribute to disease progression by suppressing effector T cell function.
By targeting CD122, therapeutic strategies can either enhance or block Treg function depending on the disease context. For instance, blocking CD122 with antibodies such as TM-β1 in autoimmune settings can reduce Treg-mediated suppression, allowing the immune system to fight off the aberrant autoimmune response.
CD122 as a Therapeutic Target: Antibodies and Immune Modulation
TM-β1 and Its Role in Immunotherapy
TM-β1 is a monoclonal antibody that targets the IL-2Rβ (CD122) subunit. Its mechanism of action can either inhibit or enhance IL-2 and IL-15 signaling, making it versatile for both cancer treatment and autoimmune disease management. TM-β1’s specificity allows it to precisely modulate T cell activity, making it a potent tool for fine-tuning immune responses.
- In cancer therapy: By enhancing CD122 signaling, TM-β1 can promote the activation and expansion of CD8+ T cells and NK cells, leading to improved tumor destruction. It is particularly effective in boosting immune responses in patients undergoing checkpoint inhibitor therapy,
where the goal is to release the "brakes" on the immune system. - In autoimmune diseases: Conversely, in diseases characterized by overactive immune responses, blocking CD122 can suppress T cell proliferation and reduce inflammation. This approach has been investigated in conditions such as type 1 diabetes, psoriasis, and systemic lupus erythematosus (SLE), where controlling excessive T cell activity is essential to limit tissue damage.
Clinical Applications of CD122 Modulation
Therapeutic Context | Mechanism of Action | Clinical Outcome |
|---|---|---|
Enhance IL-2/IL-15 signaling via CD122 to boost T cell activity | Increased tumor cytotoxicity, improved survival | |
Block IL-2/IL-15 signaling via CD122 to suppress overactive T cells | Reduced inflammation and tissue destruction | |
Enhanced viral clearance, long-term immunity |
Challenges and Future Directions in CD122-Targeted Therapies
While targeting CD122 presents a promising avenue in immunotherapy, there are several challenges to overcome:
- Balancing immune activation: Enhancing CD122 signaling, especially with IL-2, can lead to cytokine release syndrome (CRS), a severe and potentially life-threatening immune reaction. Optimizing the dosage and delivery method of anti-CD122 therapies is critical to minimize these risks.
- Selective targeting of effector cells: As IL-2 also expands regulatory T cells, therapies must selectively boost effector T cells without activating Tregs. This balance is key to maximizing the efficacy of cancer immunotherapies while minimizing immune suppression.
- Long-term immune modulation: In autoimmune diseases, prolonged suppression of CD122 signaling may compromise the immune system’s ability to respond to infections. Careful monitoring of immune status is essential to prevent unintended immunosuppression during treatment.
Advances in CD122-Targeting Strategies
Emerging technologies in antibody engineering and cytokine delivery systems offer ways to improve the safety and specificity of CD122-targeted therapies. For example, bi-specific antibodies that target both CD122 and another receptor can provide more controlled immune activation. Additionally, nanoparticle-based cytokine delivery could allow for more precise targeting of CD122-expressing cells, reducing off-target effects.
Conclusion
CD122 plays a central role in regulating T cell responses, making it a key target in both cancer and autoimmune immunotherapies. Through careful modulation of CD122 signaling, therapies can be tailored to enhance anti-tumor immunity or suppress autoimmune reactions. Monoclonal antibodies like TM-β1 demonstrate significant potential in fine-tuning immune responses, offering hope for more effective and personalized treatments in the future.
References
- Boyman, O., Sprent, J., 2012. The role of interleukin-2 during homeostasis and activation of the immune system. Nature Reviews Immunology, 12(3), pp.180-190.
- Waldmann, T.A., 2006. The biology of interleukin-15 and its receptor. Nature Reviews Immunology, 6(11), pp.835-846.
- Liao, W., Lin, J.X., Leonard, W.J., 2011. Interleukin-2 at the crossroads of effector responses,
tolerance, and immunotherapy. Immunity, 38(1), pp.13-25. - Grabstein, K.H., et al., 1994. IL-15: a novel T cell growth factor that shares activities and receptor components with IL-2. The Journal of Immunology, 152(12), pp.5770-5781.
- Ma, A., Koka, R., Burkett, P., 2006. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annual Review of Immunology, 24(1), pp.657-679.
- Gagnon, J., et al., 2008. IL-2 receptor-beta-dependent STAT5 activation is negatively regulated by T cell antigen receptor signaling. The Journal of Immunology, 180(5), pp.2538-2547.
- Klatzmann, D., Abbas, A.K., 2015. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nature Reviews Immunology, 15(5), pp.283-294.
- Fehérvari, Z., Sakaguchi, S., 2004. CD25+CD4+ regulatory T cells in the control of autoimmunity. Annual Review of Immunology, 22(1), pp.531-562.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025