TLR4: Unraveling the Role of Inflammation in Cancer Progression
Introduction to TLR4 and Cancer
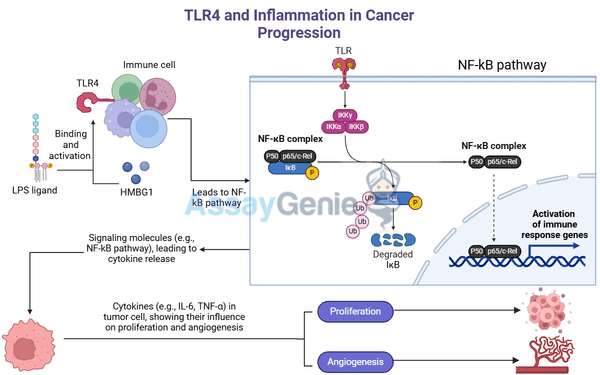
Toll-like receptor 4 (TLR4) is a key component of the innate immune system, primarily responsible for detecting infections and initiating inflammatory responses. This receptor recognizes pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharides (LPS) from bacteria, and damage-associated molecular patterns (DAMPs) from injured or stressed cells. Once activated, TLR4 triggers the release of pro-inflammatory cytokines, which are crucial for defending the body against infections.
However, the role of TLR4 in cancer is more complex. While TLR4-mediated inflammation is essential for immune protection, chronic inflammation driven by TLR4 activation in the tumor microenvironment (TME) can promote tumor growth, metastasis, and immune evasion. The TLR4 pathway is a double-edged sword: it can initiate anti-tumor immune responses, but prolonged activation of TLR4 often creates an immunosuppressive environment that supports tumor progression.
UT41, a monoclonal antibody designed to block TLR4, represents a new approach in cancer immunotherapy. By targeting TLR4, therapies like UT41 aim to reduce the tumor-promoting effects of chronic inflammation and enhance the body’s immune response to cancer.
TLR4: Structure and Function in Inflammation
The TLR4 Signaling Pathway
TLR4 is a pattern recognition receptor (PRR) that triggers immune responses upon recognizing PAMPs from pathogens and DAMPs released by damaged or dying cells. TLR4 is activated by binding to LPS or DAMPs such as HMGB1 and HSPs. Once activated, TLR4 initiates two main signaling pathways:
- MyD88-dependent pathway: This pathway leads to the activation of NF-κB, a transcription factor that drives the production of pro-inflammatory cytokines like IL-6, TNF-α, and IL-1β.
- TRIF-dependent pathway: This branch of TLR4 signaling results in the activation of IRF3, leading to the production of type I interferons (e.g., IFN-β), which are involved in antiviral responses and inflammation.
The MyD88 and TRIF pathways coordinate the immune response to infections and tissue damage, promoting the recruitment of immune cells like macrophages, dendritic cells, and neutrophils to the site of inflammation.
Chronic Inflammation and Cancer Development
While acute TLR4 activation helps to eliminate pathogens and repair tissue, chronic TLR4 activation—often seen in the tumor microenvironment—can promote cancer development. Persistent TLR4 signaling leads to:
- Tumor-promoting inflammation: Prolonged production of cytokines like IL-6 and TNF-α supports tumor cell survival and proliferation.
- Angiogenesis: Inflammatory cytokines stimulate the formation of new blood vessels, ensuring that tumors receive a continuous supply of oxygen and nutrients.
- Metastasis: TLR4 activation enhances the expression of matrix metalloproteinases (MMPs) and promotes epithelial-to-mesenchymal transition (EMT), allowing cancer cells to invade surrounding tissues and spread to distant organs.
Chronic inflammation driven by TLR4 is implicated in several cancers, including colorectal cancer, breast cancer, lung cancer, and hepatocellular carcinoma (liver cancer).
The Dual Role of TLR4 in Cancer: Tumor Promotion vs. Immune Activation
TLR4 in Tumor Progression
In the context of cancer, TLR4 has a dual role. While it can activate anti-tumor immunity in some cases, chronic TLR4 activation often facilitates tumor progression by creating an environment that promotes immune evasion. The pro-tumor effects of TLR4 include:
- Proliferation of cancer cells: Inflammatory cytokines produced in response to TLR4 activation stimulate cancer cell growth.
- Immune suppression: Chronic TLR4 signaling leads to the recruitment of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), which suppress anti-tumor immune responses.
- Resistance to apoptosis: TLR4 activation can make tumor cells more resistant to programmed cell death, allowing them to survive and proliferate even under stress.
TLR4 in Anti-Tumor Immunity
In some contexts, TLR4 activation can promote anti-tumor immunity. For example, when TLR4 is activated in dendritic cells, it enhances their ability to present antigens to T cells, leading to the activation of cytotoxic T lymphocytes (CTLs) that target and kill tumor cells. Similarly, TLR4 activation can stimulate the release of type I interferons, which support the immune system’s ability to fight cancer.
This complex and context-dependent role of TLR4 highlights the potential of modulating TLR4 activity in cancer therapy—specifically, blocking its tumor-promoting effects while preserving or enhancing its immune-activating properties.
Targeting TLR4 with UT41: A Novel Therapeutic Approach
UT41: A Monoclonal Antibody Targeting TLR4
UT41 is a monoclonal antibody designed to block TLR4 activation, preventing its pro-inflammatory and tumor-promoting effects. By inhibiting TLR4 signaling, UT41 can:
- Reduce chronic inflammation: By blocking TLR4, UT41 prevents the release of pro-inflammatory cytokines like TNF-α and IL-6, which contribute to tumor growth and metastasis.
- Suppress tumor progression: Inhibiting TLR4 disrupts the inflammatory environment that supports tumor survival, reducing the tumor’s ability to grow and spread.
- Enhance immune responses: In certain cases, blocking TLR4 may restore the immune system’s ability to recognize and attack cancer cells, improving the effectiveness of other cancer treatments like checkpoint inhibitors.
Mechanism of Action of UT41
UT41 works by binding to TLR4 and preventing its activation by LPS or tumor-derived DAMPs. This inhibition disrupts the downstream signaling pathways that promote inflammation and tumor growth. The key effects of UT41 include:
- Blocking pro-inflammatory signaling: By inhibiting TLR4 activation, UT41 reduces the production of cytokines like TNF-α, IL-6, and IL-1β.
- Suppressing tumor-supportive inflammation: UT41 helps to break the cycle of chronic inflammation that fuels tumor progression.
- Reducing immunosuppressive cell recruitment: By preventing TLR4-driven inflammation, UT41 reduces the recruitment of Tregs and MDSCs, both of which suppress anti-tumor immune responses.
Clinical Applications of UT41
UT41 has potential applications in cancers where chronic inflammation plays a central role in driving tumor progression, including:
- Colorectal cancer: Chronic inflammation in the gut, often caused by inflammatory bowel
disease (IBD), can activate TLR4, promoting colorectal cancer development. UT41 could reduce inflammation and slow tumor growth. - Lung cancer: TLR4 is activated in response to environmental toxins and smoking, contributing to lung cancer progression. Blocking TLR4 with UT41 may reduce inflammation-driven tumor growth.
- Hepatocellular carcinoma: Liver inflammation caused by viral infections (e.g., hepatitis B and C) or alcohol abuse activates TLR4, promoting liver cancer. UT41 could potentially disrupt this inflammation and slow disease progression.
Cancer Type | TLR4 Activation | Potential of TLR4 Blockade (UT41) |
|---|---|---|
TLR4 activation by DAMPs and gut inflammation | UT41 can reduce inflammation and limit tumor growth and metastasis. | |
TLR4 activated by smoking and toxins | UT41 may reduce chronic inflammation and slow tumor progression. | |
Inflammation from hepatitis infections activates TLR4 | UT41 could reduce liver inflammation and enhance immune responses against the tumor. |
Synergy with Other Immunotherapies
Combination with Checkpoint Inhibitors
The immunosuppressive effects of TLR4-driven inflammation can limit the effectiveness of checkpoint inhibitors like anti-PD-1 and anti-CTLA-4. By blocking TLR4, UT41 may reduce chronic inflammation and improve the immune system’s ability to recognize and attack tumor cells, enhancing the therapeutic efficacy of checkpoint inhibitors.
Potential for Combining UT41 with Chemotherapy
Chemotherapy often leads to the release of DAMPs from dying tumor cells, which can activate TLR4 and promote resistance to treatment. UT41 could help counteract this effect by preventing DAMPs from activating TLR4, making chemotherapy more effective.
Challenges and Future Directions in TLR4-Targeted Therapy
Managing Immune-Related Toxicities
While targeting TLR4 holds promise for reducing inflammation-driven tumor progression, there is a risk of immune-related toxicities due to the critical role TLR4 plays in immune defense. Blocking TLR4 could impair the body’s ability to fight infections, making careful monitoring and patient selection essential.
Expanding the Use of TLR4 Inhibitors
Future research will likely explore the broader applicability of TLR4-targeting therapies beyond inflammation-driven cancers. Identifying biomarkers that predict patient response to TLR4 blockade will be crucial for expanding its use in combination with other treatments.
Conclusion
TLR4 plays a complex role in cancer, contributing to both inflammation-driven tumor progression and immune activation. Targeting TLR4 with therapies like UT41 offers a promising strategy to reduce chronic inflammation, disrupt tumor-promoting processes, and enhance the immune system’s ability to fight cancer. As research continues, TLR4-targeted therapies may become an important component of combination immunotherapy strategies for treating cancer.
References
- Rakoff-Nahoum, S., Medzhitov, R., 2009. Toll-like receptors and cancer. Nature Reviews Cancer, 9(1), pp.57-63.
- Kawai, T., Akira, S., 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology, 11(5), pp.373-384.
- Pradere, J.P., Dapito, D.H., Schwabe, R.F., 2014. The Yin and Yang of Toll-like receptors in cancer. Oncogene, 33(27), pp.3485-3495.
- Huang, B., Zhao, J., Unkeless, J.C., et al., 2008. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene, 27(2), pp.218-224.
- Takeda, K., Akira, S., 2015. Toll-like receptors. Current Protocols in Immunology, 109(1), pp.14-18.
- Yu, L., Chen, S., 2008. Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunology, Immunotherapy, 57(9), pp.1271-1278.
- Li, N., 2014. TLR4 as the therapeutic target in chemotherapy resistance. Cancer Biology & Therapy, 15(3), pp.1-9.
Recent Posts
-
Tigatuzumab Biosimilar: Harnessing DR5 for Targeted Cancer Therapy
Tigatuzumab is a monoclonal antibody targeting death receptor 5 (DR5), a member of the …17th Dec 2025 -
Enavatuzumab Biosimilar: Advancing TWEAKR-Targeted Therapy in Cancer
Enavatuzumab is a monoclonal antibody targeting TWEAK receptor (TWEAKR, also known as …17th Dec 2025 -
Alemtuzumab Biosimilar: Advancing CD52-Targeted Therapy
Alemtuzumab is a monoclonal antibody targeting CD52, a glycoprotein highly expressed o …17th Dec 2025