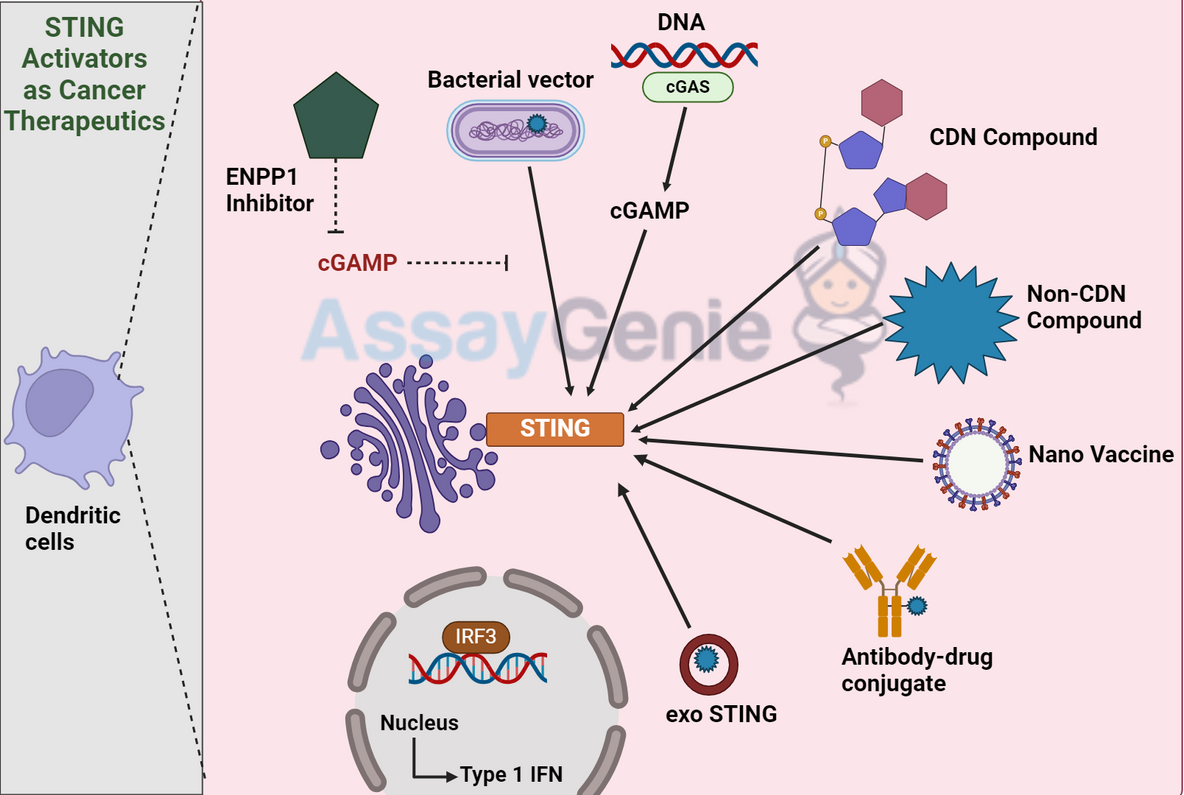
STING Activators As Cancer Therapeutics
The STING (Stimulator of Interferon Genes) pathway plays a pivotal role in the innate immune system's response to cancerous cells and DNA viruses. Exploiting this pathway through STING activators presents a promising avenue for cancer therapeutics. This article delves into the mechanism of action of STING activators, their therapeutic potential, challenges in their development, and the latest advancements in the field.
Understanding the STING Pathway
The Biological Role of STING
The STING pathway is integral to the innate immune response, detecting cytosolic DNA to trigger the production of type I interferons and other cytokines. This response is crucial for the immune system's ability to combat infections and recognize malignant cells.
Mechanism of STING Activation
STING activation occurs through the sensing of cyclic dinucleotides (CDNs), which are either produced endogenously or introduced externally. Upon binding to these CDNs, STING undergoes a conformational change, initiating a signaling cascade that culminates in the activation of transcription factors such as IRF3 and NF-κB, leading to the production of type I interferons and pro-inflammatory cytokines.
STING Activators in Cancer Therapy
Rationale for Using STING Activators
The rationale behind using STING activators in cancer therapy lies in their ability to induce a robust immune response against tumors. By activating the STING pathway, these agents can enhance the visibility of cancer cells to the immune system, promoting their elimination.
Types of STING Activators
STING activators hold significant therapeutic potential, demonstrated by their ability to shrink tumors in preclinical models. However, challenges such as ensuring selective targeting of tumor cells, avoiding systemic inflammation, and overcoming the immunosuppressive tumor microenvironment remain.
Advances in STING Activator Development
Novel STING Activators and Their Mechanisms
Recent advancements in the field have led to the development of novel STING activators with improved pharmacokinetic properties and enhanced specificity for the STING pathway. These include modifications to the CDN backbone and the development of non-nucleotide STING agonists.
Clinical Trials and Results
Several STING activators are currently undergoing clinical trials, with early results showing promise in terms of safety and initial efficacy. These studies are crucial for determining the optimal dosing regimens, administration routes, and combinations with other cancer therapies.
Overcoming Resistance and Enhancing Efficacy
Innovations and research directions aimed at overcoming current challenges, such as combination therapies, biomarker discovery, and novel checkpoint targets, are discussed, shedding light on the future of cancer treatment.
Table: Key STING Activators in Development
| Activator Name | Type | Mechanism of Action | Clinical Trial Phase |
| Compound A | Synthetic CDN | Mimics endogenous CDNs, activating STING directly | Phase I |
| Compound B | Non-nucleotide agonist | Binds to STING, inducing a conformational change | Phase II |
| Compound C | Modified CDN | Enhanced stability and specificity for STING | Preclinical |
Conclusion
STING activators represent a novel class of cancer therapeutics with the potential to revolutionize the treatment of various cancers. While challenges remain in their development and clinical application, ongoing research and clinical trials are rapidly advancing our understanding and ability to harness the STING pathway for cancer therapy.
References
- Barber, G.N. (2015). STING: infection, inflammation and cancer. Nature Reviews Immunology, 15(12), 760-770.
- Corrales, L., et al. (2015). STING activation for cancer immunotherapy. Trends in Immunology, 36(11), 677-684.
- Deng, L., et al. (2014). STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity, 41(5), 843-852.
- Miao L, Qi J, Zhao Q, Wu QN, Wei DL, Wei XL, Liu J, Chen J, Zeng ZL, Ju HQ, Luo HY, Xu RH. Targeting the STING pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics. 2020 Jan 1;10(2):498-515. doi: 10.7150/thno.37745. PMID: 31903134; PMCID: PMC6929973.
- Ohkuri, T., et al. (2014). STING contributes to antitumor immunity in a syngeneic mouse model. Cancer Immunology Research, 2(5), 403-414.
- Ribas, A., Wolchok, J.D. (2018). Cancer immunotherapy using checkpoint blockade. Science, 359(6382), 1350-1355.
- Wilson, E.B., et al. (2013). Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science, 340(6129), 202-207. 8. Gan Y, Li X, Han S, Liang Q, Ma X, Rong P, Wang W, Li W. The cGAS/STING Pathway: A Novel Target for Cancer Therapy. Front Immunol. 2022 Jan 3;12:795401. doi: 10.3389/fimmu.2021.795401. PMID: 35046953; PMCID: PMC8761794.
Written by Zainab Riaz
Zainab Riaz completed her Master degree in Zoology from Fatimah Jinnah University in Pakistan and is currently pursuing a Doctor of Philosophy in Zoology at University of Lahore in Pakistan.
Recent Posts
-
Biological Role of GLP-1
Glucagon-like peptide-1 (GLP-1) is a critical hormone in the regulation of glucose met …20th Jun 2024 -
Th17 Cell Differentiation: Insights into Immunological Dynamics
Th17 cells, a subset of T helper cells characterized by their production of interleukin-17 (IL- …25th May 2024 -
Assay Genie New Asian Distributor May 2024
Dublin, Ireland — May 20th 2024 — Assay Genie, a leading supplier of ELISA Kits, Ant …19th May 2024