PVR: Balancing Immune Activation and Suppression in Cancer
PVR (Poliovirus receptor), also known as CD155, is a molecule that plays a dual role in regulating the immune response within the tumor microenvironment. While initially discovered as a receptor for poliovirus, PVR is now recognized for its involvement in modulating immune cell interactions, particularly in cancer. Its expression on tumor cells can both activate and suppress the immune system, making it an intriguing target for cancer immunotherapy. Antibodies such as Anti-PVR (e.g., D172) are being explored as potential therapeutic agents to restore immune function and improve tumor clearance.
PVR: An Overview of Its Role in the Immune System
What Is PVR (CD155)?
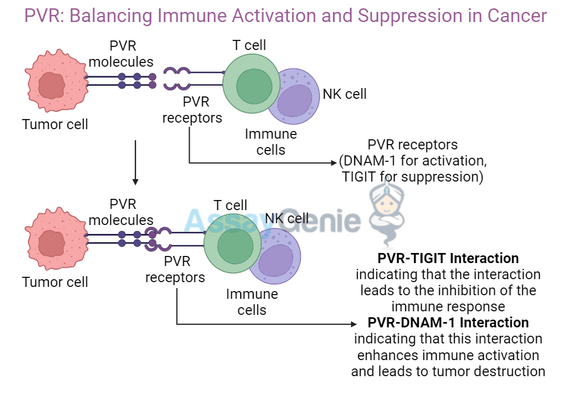
PVR is a transmembrane glycoprotein that belongs to the nectin family. It is widely expressed on various cell types, including tumor cells. In the context of cancer, PVR interacts with two key receptors on immune cells:
- DNAM-1 (CD226) – Promotes T cell and NK cell activation.
- TIGIT – Suppresses immune responses by binding to PVR, leading to immune evasion.
This dual role highlights PVR's complexity in cancer biology.
Mechanisms of PVR in Immune Activation and Suppression
PVR-Mediated Immune Activation
PVR engages DNAM-1 on T cells and natural killer (NK) cells, promoting their activation. When bound to DNAM-1, PVR facilitates:
- T cell proliferation and cytotoxicity.
- NK cell activation, leading to the destruction of tumor cells.
- Cytokine production, such as IFN-γ, enhancing the overall immune response against tumors.
Interaction | Effect on Immune Cells |
|---|---|
Increases T cell and NK cell activation, enhancing tumor clearance. |
PVR-Mediated Immune Suppression
Conversely, PVR also binds to TIGIT, an inhibitory receptor on T cells and NK cells, which suppresses immune activity. This allows tumor cells to escape immune detection by:
- Inhibiting T cell responses.
- Reducing NK cell cytotoxicity.
- Decreasing the production of anti-tumor cytokines.
Interaction | Effect on Immune Cells |
|---|---|
Suppresses T cell and NK cell functions, aiding tumor immune evasion. |
Anti-PVR Antibodies: Restoring Immune Balance in Cancer
Anti-PVR (e.g., D172): Mechanism of Action
Anti-PVR antibodies, such as D172, are designed to block the interaction between PVR and its receptors, particularly TIGIT, while preserving the PVR-DNAM-1 interaction. This selective targeting can:
- Enhance T cell activation by preventing PVR from binding to TIGIT.
- Boost NK cell-mediated tumor clearance by promoting DNAM-1 signaling.
- Restore immune balance in the tumor microenvironment, shifting the response from suppression to activation.
Antibody | Target Mechanism | Outcome |
|---|---|---|
D172 | Blocks PVR-TIGIT interaction while enhancing PVR-DNAM-1 binding | Increases T cell and NK cell activity, promoting tumor clearance. |
Therapeutic Potential of Anti-PVR in Immunotherapy
Blocking PVR-TIGIT interactions while preserving PVR-DNAM-1 activity offers a promising strategy in cancer immunotherapy. By restoring immune activation in the tumor microenvironment, Anti-PVR antibodies can:
- Overcome tumor-induced immune suppression.
- Enhance the effectiveness of existing therapies, such as checkpoint inhibitors.
- Improve patient outcomes, especially in cancers with high PVR expression.
PVR as a Target in Combination Therapies
Combining Anti-PVR with Checkpoint Inhibitors
PVR’s role in suppressing immune responses makes it a promising target for combination with other immunotherapies, such as PD-1/PD-L1 inhibitors. By inhibiting both TIGIT and PD-1 pathways, a synergistic effect can be achieved, leading to:
- Greater immune activation and reduced tumor immune evasion
- Increased T cell infiltration into tumors, boosting the chances of tumor rejection.

Combination Therapy | Expected Synergistic Effect |
|---|---|
Anti-PVR + Anti-PD-1/PD-L1 | Overcomes multiple immune checkpoints, amplifying anti-tumor responses. |
Enhancing NK Cell Function in Tumor Clearance
Future Directions: Anti-PVR in Cancer Immunotherapy
Ongoing Clinical Trials and Research
Preclinical studies and early-phase clinical trials involving Anti-PVR antibodies like D172 are investigating their safety and efficacy in various cancers, particularly those with high PVR expression. These trials aim to:
- Determine optimal dosing strategies.
- Assess long-term immune responses and tumor regression.
- Explore synergies with other immunotherapies to improve patient outcomes.
Clinical Trial Phase | Focus | Preliminary Findings |
|---|---|---|
Phase I/II | Safety and efficacy of Anti-PVR (e.g., D172) | Increased T cell and NK cell activity in solid tumors. |
Conclusion: Targeting PVR to Balance Immune Responses
PVR plays a pivotal role in balancing immune activation and suppression within the tumor microenvironment. By blocking the inhibitory effects of PVR-TIGIT interactions and promoting PVR-DNAM-1 signaling, Anti-PVR therapies, such as D172, offer a promising approach to enhance T cell and NK cell responses against tumors. As research progresses, Anti-PVR antibodies could become an integral part of combination immunotherapies, offering new hope for patients with immune-resistant cancers.
References
- Johnston, R.J., Comps-Agrar, L., Hackney, J., Yu, X., Huseni, M., Yang, Y., Park, S., Javinal, V., Chiu, H., Irving, B.A., Eaton, D.L. and Grogan, J.L. (2014) 'The immunoreceptor TIGIT regulates antitumor and antiviral CD8⁺ T cell effector function', Cancer Cell, 26(6), pp. 923–937.
- Li, X.Y., Das, I., Lepletier, A., Addala, V., Bald, T., Stannard, K., Barkauskas, D.S., Liu, J., Aguilera, A.R., Takeda, K., Smyth, M.J., Martinet, L. and Chesi, M. (2019) 'CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms', The Journal of Clinical Investigation, 129(5), pp. 2068-2084.
- Masson, D., Jarry, A., Baury, B., Blanchardie, P., Laboisse, C.L., Lustenberger, P., Tréhoux, S., Denis, M.G., Staedel, C. and Volant, A. (2001) 'Overexpression of the CD155 gene in human colorectal carcinoma', Gut, 49(2), pp. 236-240.
- Martinet, L. and Smyth, M.J. (2015) 'Balancing natural killer cell activation through paired receptors', Nature Reviews Immunology, 15(4), pp. 243-254.
- Stanietsky, N., Simic, H., Arapovic, J., Toporik, A., Levy, O., Novitsky, S.G., Levine, Z., Beiman, M., Dassa, L., Achdout, H., Stern-Ginossar, N., Jonjic, S., Seidel, E. and Porgador, A. (2009) 'The interaction of TIGIT with PVR and its implications for NK cell-mediated immune evasion of tumors', Proceedings of the National Academy of Sciences of the United States of America, 106(42), pp. 17858-17863.
- Wang, P.L., O'Farrell, S., Clayberger, C. and Krensky, A.M. (2009) 'Identification and molecular cloning of TIGIT, a novel immunoreceptor and coinhibitory molecule', European Journal of Immunology, 39(3), pp. 782-795.
- Zhang, Q., Bi, J., Zheng, X., Chen, Y., Wang, H., Wu, W., Wang, Z., Wu, Q., Peng, H., Wei, H. and Sun, R. (2018) 'Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity', Nature Immunology, 19(7), pp. 723-732.
Recent Posts
-
Tigatuzumab Biosimilar: Harnessing DR5 for Targeted Cancer Therapy
Tigatuzumab is a monoclonal antibody targeting death receptor 5 (DR5), a member of the …17th Dec 2025 -
Enavatuzumab Biosimilar: Advancing TWEAKR-Targeted Therapy in Cancer
Enavatuzumab is a monoclonal antibody targeting TWEAK receptor (TWEAKR, also known as …17th Dec 2025 -
Alemtuzumab Biosimilar: Advancing CD52-Targeted Therapy
Alemtuzumab is a monoclonal antibody targeting CD52, a glycoprotein highly expressed o …17th Dec 2025