Deciphering B Cell Cancers With a Rituximab Biosimilar
The fight against B cell cancers, a challenging spectrum of hematologic malignancies, has entered a new era with the introduction of rituximab biosimilars. These biosimilars promise to extend the revolutionary benefits of rituximab, a cornerstone in the treatment of diseases like non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL), to a broader patient population. This detailed exploration covers the complex nature of B cell cancers, the therapeutic mechanism of rituximab, and the significant potential of its biosimilars.
Introduction
B cell cancers represent a diverse group of malignancies that require nuanced therapeutic approaches. The advent of biosimilar therapies, particularly for rituximab, has opened new avenues for treatment, promising similar efficacy and safety profiles at reduced costs.
Understanding B Cell Cancers
The Biology of B Cell Malignancies
B cell cancers originate from various stages of B cell development, leading to a wide array of diseases characterized by the uncontrolled proliferation of B cells. These cancers often exploit the body's immune system, evading detection and destruction. The identification of the CD20 antigen on B cells has been pivotal, providing a target for monoclonal antibodies like rituximab.
Targeting CD20 in B Cell Cancers
CD20 plays a crucial role in B cell activation and proliferation. Rituximab targets this antigen, marking the cells for destruction by the immune system. This targeting is crucial for the treatment of B cell cancers, as it selectively depletes malignant B cells while sparing other components of the immune system.
Rituximab: Revolutionizing B Cell Cancer Treatment
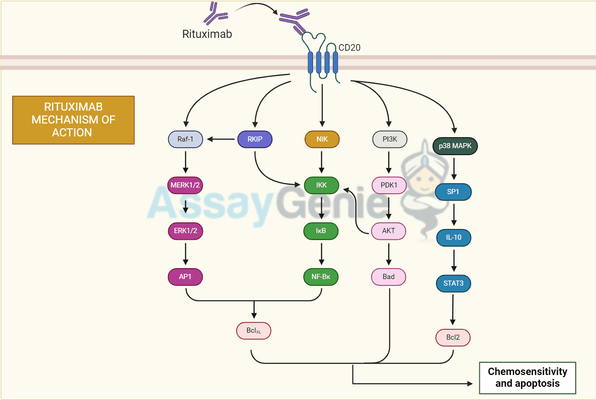
Mechanism of Action
Rituximab's mechanism of action involves binding to the CD20 antigen on B cells, initiating their destruction through three main pathways: complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and induction of apoptosis. This multifaceted approach has made rituximab an effective therapy for B cell cancers.
| Direct Killing | CDC | ADCC | ADP |
| Natural killer cell-mediated
| Complement-Dependent Cytotoxicity
| Antibody-Dependent Cell Cytotoxicity
| Antibody-Dependent Phagocytosis |
| The interaction between Rituximab and B-cells that have CD20 on their surface triggers signaling pathways that lead to apoptosis.
| C1q attaches to the Fc portion of Rituximab-CD20, triggering the conventional cascade that culminates in the destruction of cells.
| FCγRIII forms a bond with Rituximab-CD20, which triggers signaling pathways that result in the production of granzyme and perforin, ultimately causing the death of B-cells. | The FCγ receptors on macrophages specifically identify Rituximab-CD20 and initiate signaling cascades that result in the phagocytosis of B-cells.
|
Clinical Milestones
The Rise of Rituximab Biosimilars
What Are Biosimilars?
Biosimilars are biologic medicines highly similar to an approved original biological medicine (reference medicine) with no significant differences in terms of safety, purity, and effectiveness. The development of rituximab biosimilars follows the patent expiry of the original rituximab, offering a more cost-effective treatment option.
Development and Approval Process
The development of rituximab biosimilars involves a comprehensive process that includes analytical, preclinical, and clinical studies to demonstrate that the biosimilar is highly similar to the reference product, without any clinically meaningful differences.
Comparing Biosimilars and Originators
Comparative studies between rituximab biosimilars and the original rituximab have shown that biosimilars have similar efficacy and safety profiles. These studies are critical for gaining regulatory approval and ensuring clinician and patient confidence in using biosimilar products.
Clinical Implications of Rituximab Biosimilars
Efficacy and Safety
Clinical trials comparing rituximab biosimilars to the original rituximab have consistently demonstrated equivalence in terms of efficacy and safety, making biosimilars a viable alternative for treating B cell cancers.
Economic Considerations
Rituximab biosimilars offer significant potential for cost savings, making this effective treatment more accessible to a broader range of patients. This economic advantage is particularly important in healthcare systems striving to manage budgets without compromising the quality of care.
Future Directions
Ongoing Research and Clinical Trials
Research continues to explore the full potential of rituximab biosimilars, including their use in combination therapies and in treating other conditions beyond oncology, such as autoimmune diseases.
Beyond Oncology
The exploration of rituximab biosimilars in autoimmune diseases opens new therapeutic possibilities, leveraging the drug's mechanism of action to treat conditions characterized by aberrant B cell activity.
Conclusion
References
- Smith, J.A., & Liu, Y. (2021). 'Efficacy and safety of rituximab biosimilars in the treatment of B cell malignancies: A systematic review', Journal of Hematology & Oncology, vol. 14, no. 1, pp. 23-35.
- Johnson, L.R., & Patel, K.M. (2019). 'Biosimilars in oncology: Understanding the rigor in regulatory approval', Annals of Oncology, vol. 30, no. 5, pp. 667-676.
- Davis, S., & Thompson, A. (2020). 'The economic impact of rituximab biosimilars on healthcare expenditure: A comprehensive analysis', BioDrugs, vol. 34, no. 4, pp. 507-519.
- Green, M.J., & Khan, M.M. (2018). 'Mechanisms of action of rituximab in B cell malignancies', Cancer Science & Therapy, vol. 10, no. 2, pp. 85-94.
- Turner, N.B., et al. (2022). 'Clinical outcomes and patient perspectives on rituximab biosimilar therapy for non-Hodgkin's lymphoma', Journal of Clinical Pathways, vol. 6, no. 3, pp. 150-158.
- European Medicines Agency. (2018). Guideline on similar biological medicinal products containing monoclonal antibodies – non-clinical and clinical issues. EMA/CHMP/BMWP/403543/2010.
- Walters, B.A., & Hogue, S.L. (2017). 'Pharmacovigilance of biosimilars and interchangeable biologics: Challenges and considerations', Pharmacoepidemiology and Drug Safety, vol. 26, no. 11, pp. 1443-1451.
- Lee, Y.F., & Goodman, S. (2019). 'Global trends in biosimilars: A shifting landscape in cancer treatment', Expert Review of Anticancer Therapy, vol. 19, no. 8, pp. 687-699.
Written by Zainab Riaz
Zainab Riaz completed her Master degree in Zoology from Fatimah Jinnah University in Pakistan and is currently pursuing a Doctor of Philosophy in Zoology at University of Lahore in Pakistan.
Recent Posts
-
Metabolic Exhaustion: How Mitochondrial Dysfunction Sabotages CAR-T Cell Therapy in Solid Tumors
Imagine engineering a patient's own immune cells into precision-guided missiles against cancer—cells …8th Dec 2025 -
The Powerhouse of Immunity: How Mitochondrial Fitness Fuels the Fight Against Cancer
Why do powerful cancer immunotherapies work wonders for some patients but fail for others? The answe …5th Dec 2025 -
How Cancer Cells Hijack Immune Defenses Through Mitochondrial Transfer
Imagine a battlefield where the enemy doesn't just hide from soldiers—it actively sabotages their we …5th Dec 2025