Brentuximab Biosimilar: Advancing CD30-Targeted Therapy
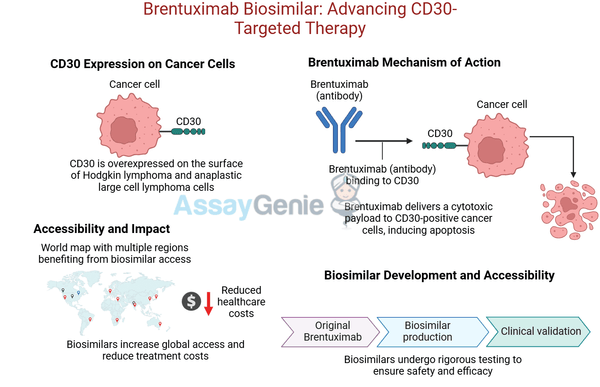
Brentuximab vedotin is a CD30-targeting antibody-drug conjugate (ADC) approved for treating CD30-positive malignancies, such as Hodgkin lymphoma (HL) and anaplastic large cell lymphoma (ALCL). This ADC combines a monoclonal antibody targeting CD30 with a cytotoxic payload, monomethyl auristatin E (MMAE), to selectively kill cancer cells. The biosimilar HDBS0017 offers the same clinical efficacy and safety as the original biologic at a reduced cost, increasing access to this life-saving therapy.
1. Understanding CD30 and Its Role in Cancer
CD30 in Malignancies
CD30 is a cell surface protein expressed on:
- Reed-Sternberg cells in classical Hodgkin lymphoma (HL).
- Activated T and B cells in ALCL.
- Other CD30-positive malignancies, such as cutaneous T-cell lymphoma (CTCL).
CD30 is minimally expressed in normal cells, making it an ideal target for antibody-drug conjugate therapy.
2. HDBS0017: A Cost-Effective Biosimilar
Features of HDBS0017
HDBS0017 is a biosimilar to Brentuximab vedotin, providing equivalent safety, efficacy, and quality at a more affordable price.
- Target: CD30-positive malignant cells.
- Mechanism: Delivers MMAE to selectively kill tumor cells.
- Affordability: Reduces costs, improving global access to ADC therapy.
3. Mechanism of Action
Step | Details |
|---|---|
CD30 Binding | HDBS0017 binds specifically to CD30 expressed on malignant cells. |
Internalization | The antibody-CD30 complex is internalized into the tumor cell. |
MMAE Release | The cytotoxic MMAE payload is released, disrupting microtubule assembly. |
Tumor Cell Death | Microtubule disruption induces mitotic arrest and apoptosis in tumor cells. |
4. Clinical Applications
HDBS0017 is primarily indicated for the treatment of CD30-positive malignancies.
Hodgkin Lymphoma (HL)
Frontline Therapy
- HDBS0017 is used in combination with chemotherapy (e.g., AVD: doxorubicin, vinblastine, dacarbazine) for untreated HL.
- Improves progression-free survival compared to standard chemotherapy alone.
Relapsed or Refractory HL
- As monotherapy or part of salvage regimens, HDBS0017 is effective in relapsed/refractory cases following stem cell transplant or chemotherapy.
Anaplastic Large Cell Lymphoma (ALCL)
- Effective in both systemic and cutaneous forms of ALCL, achieving high response rates.
Other CD30-Positive Malignancies
- Cutaneous T-Cell Lymphoma (CTCL): Approved for CD30-expressing variants such as mycosis
fungoides. - Peripheral T-Cell Lymphoma (PTCL): Under investigation as part of combination regimens.
5. Benefits of HDBS0017
Targeted Tumor Destruction
HDBS0017 delivers its cytotoxic payload directly to CD30-positive cells, sparing healthy tissue and reducing systemic toxicity.
Cost-Effective Treatment
By lowering costs, HDBS0017 expands access to CD30-targeted therapies, particularly in low-resource settings.
Versatile Applications
Effective as monotherapy and in combination regimens, HDBS0017 is adaptable to various CD30-positive cancers and treatment settings.
6. Challenges and Considerations
Adverse Effects
- Peripheral Neuropathy: A common side effect due to MMAE, requiring dose modifications.
- Hematologic Toxicity: Includes neutropenia and thrombocytopenia, manageable with supportive care.
Resistance Development
- Downregulation of CD30 expression or increased drug efflux may lead to resistance. Combination therapies can mitigate this risk.
7. Comparison: Brentuximab vs. HDBS0017
Feature | Brentuximab Vedotin | HDBS0017 (Biosimilar) |
|---|---|---|
Target | CD30-positive tumor cells. | CD30-positive tumor cells. |
Mechanism | ADC delivering MMAE to induce mitotic arrest and apoptosis. | ADC delivering MMAE to induce mitotic arrest and apoptosis. |
Indications | HL,ALCL, CTCL, and other CD30-positive cancers. | HL, ALCL, CTCL, and other CD30-positive cancers. |
Efficacy | Proven in clinical trials. | Equivalent in preclinical and clinical studies. |
Cost | High | Reduced, improving accessibility. |
8. Future Directions
Expanded Indications
HDBS0017 is under investigation for use in additional CD30-positive malignancies and combination regimens:
- Diffuse Large B-Cell Lymphoma (DLBCL): Investigating its potential in CD30-positive subtypes.
- Pediatric Cancers: Exploring use in CD30-positive pediatric Hodgkin lymphoma and other rare malignancies.
Novel Combinations
- Checkpoint Inhibitors: Combining HDBS0017 with PD-1/PD-L1 inhibitors may enhance anti-tumor immune responses.
- Chemotherapy and Targeted Agents: Synergistic effects observed with agents like pralatrexate or romidepsin in T-cell lymphomas.
9. Summary Table
Aspect | Details |
|---|---|
Target | CD30, overexpressed in Hodgkin lymphoma, ALCL, and other malignancies. |
Primary Use | Treating CD30-positive malignancies, including HL, ALCL, and CTCL. |
Mechanism of Action | ADC delivering MMAE to induce mitotic arrest and apoptosis. |
Biosimilar Benefits | Affordable, accessible, and clinically equivalent to Brentuximab vedotin. |
Conclusion
The Brentuximab biosimilar HDBS0017 offers a transformative approach to treating CD30-positive malignancies. By combining targeted therapy with a potent cytotoxic payload, HDBS0017 provides effective and durable responses in Hodgkin lymphoma and related cancers. As a cost-effective alternative, HDBS0017 expands access to cutting-edge therapy, improving outcomes for patients worldwide.
References
- Younes, A., et al., 2018. Brentuximab vedotin in combination with chemotherapy for Hodgkin's lymphoma. NEJM, 378(4), pp.331-344.
- Pro, B., et al., 2012. Brentuximab vedotin for relapsed ALCL: Efficacy and safety. Journal of Clinical Oncology, 30(18), pp.2190-2196.
- ClinicalTrials.gov, 2023. Studies on Brentuximab vedotin and biosimilar HDBS0017. Available at www.clinicaltrials.gov.
- European Medicines Agency (EMA), 2023. Guidelines on biosimilar development for ADCs. Available at www.ema.europa.eu.
- Horwitz, S.M., et al., 2018. Targeting CD30 in T-cell lymphomas: Brentuximab vedotin and beyond. Blood Advances, 2(15), pp.1665-1673.
Recent Posts
-
Tigatuzumab Biosimilar: Harnessing DR5 for Targeted Cancer Therapy
Tigatuzumab is a monoclonal antibody targeting death receptor 5 (DR5), a member of the …17th Dec 2025 -
Enavatuzumab Biosimilar: Advancing TWEAKR-Targeted Therapy in Cancer
Enavatuzumab is a monoclonal antibody targeting TWEAK receptor (TWEAKR, also known as …17th Dec 2025 -
Alemtuzumab Biosimilar: Advancing CD52-Targeted Therapy
Alemtuzumab is a monoclonal antibody targeting CD52, a glycoprotein highly expressed o …17th Dec 2025