TREM2 is a transmembrane receptor in the immunoglobulin superfamily which has a short cytosolic tail that lacks signal transduction and trafficking motifs1. TREM2 initiates intracellular signaling by interacting with the adaptor proteins DNAX activation protein 12 (DAP12) and DAP10, which are phosphorylated to recruit signal transduction machinery when ligands bind. TREM2 interacts with a wide array of anionic ligands, including bacterial products such as lipopolysaccharide and dextran sulfates, DNA, lipoproteins, apolipoproteins, phospholipids1 and amyloid-β oligomers2.
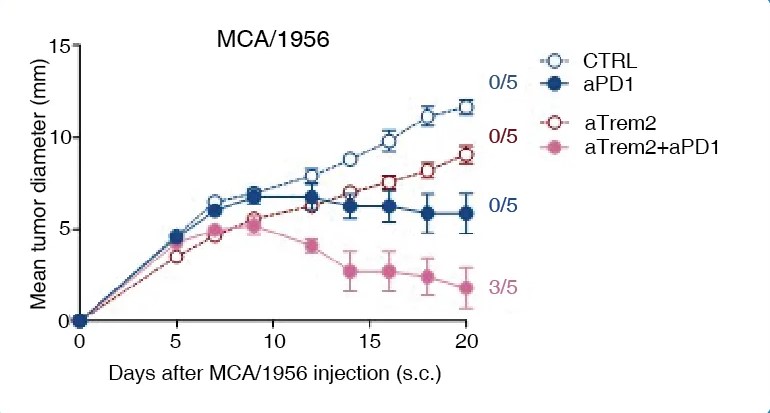
In healthy tissues, TREM2 is involved in tissue development and maintenance, synaptic pruning1, central nervous system homeostasis2, the hair follicle stem cell niche1, and activates immune remodeling when tissue damage is detected1. When dysregulated, TREM2 affects a variety of pathologies including neurodegeneration, fatty liver disease, obesity, atherosclerosis, and tumor microenvironment and development. In Alzheimer’s Disease, TREM2 activation initiates a signaling loop that promotes its own ligand production, sustains microglial responses, and leads to disease progression1, 2, 3. Defects in TREM2 also cause polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL), a fatal disease of pre-senile dementia1. Additionally, Trem2 knockout mice are more resistant to cancer growth and are more responsive to anti-PD1 immunotherapy than wild-type mice3. Given its broad role in pathology, TREM2 is a target of immunotherapy.
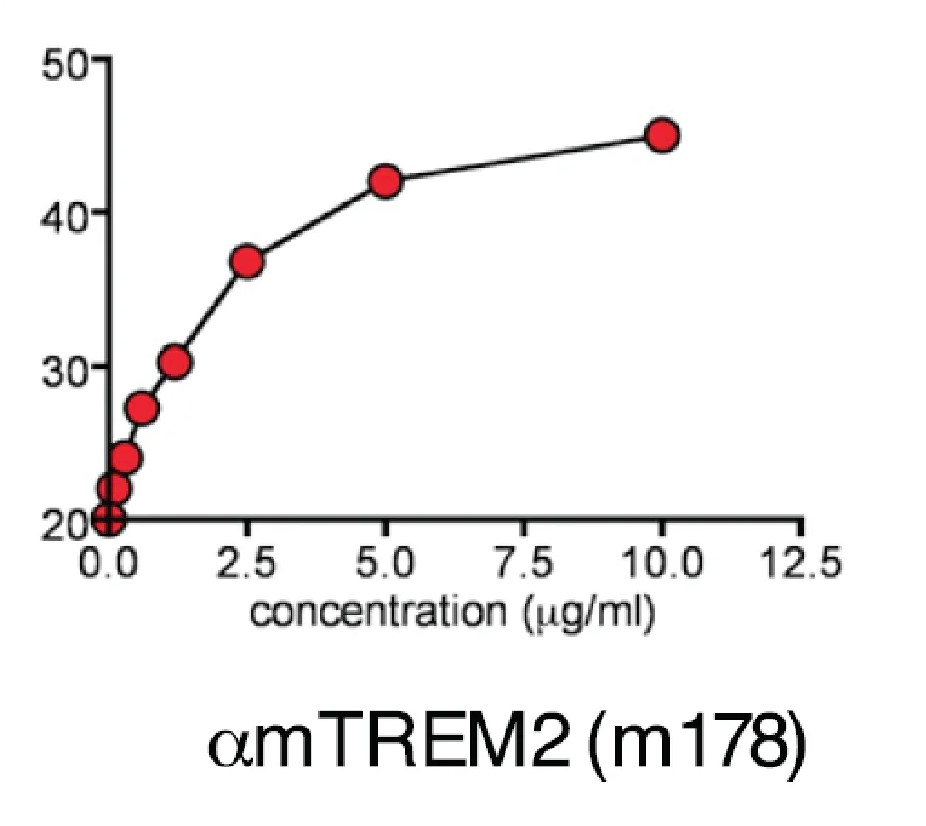
Clone 178 was generated by immunizing a Wister rat with a recombinant protein consisting of the ectodomain of TREM2 fused to the human Ig constant domain4. Spleen cells were harvested, fused with Sp2/0 myeloma cells, and the resulting hybridomas screened against Jurkat cells transiently infected with TREM2. A recombinant form of 178 was then generated, in which the variable region of the heavy chain was grafted onto a mouse IgG2a constant region backbone containing a mutated Fc domain (LALAPG)3. The LALAPG mutation prevents recognition by Fc receptors and complement, thereby minimizing antibody-dependent cellular cytotoxicity and antibody-dependent phagocytosis.
Clone 178 blocks ligand binding to TREM23 and does not cross-react with TREM14.