Description
| Product Name: | Anti-Human IFNAR1 (Anifrolumab) |
| SKU: | IVMB0523 |
| Size: | 500 µg |
| Antibody Type: | Biosimilar Recombinant Human Monoclonal Antibody |
| Clone: | MEDI-546 |
| Target: | IFNAR1 |
| Isotype: | Human IgG1κ |
| Host Species: | Human |
| Reactivity: | Human |
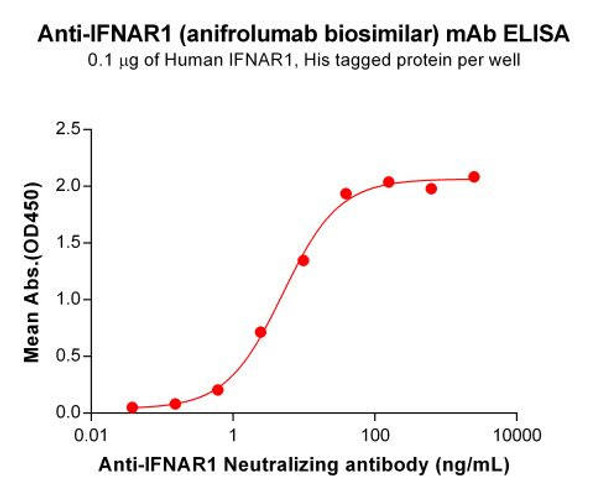
| Applications: | ELISA |
| Expression Host: | HEK-293 Cells |
| FC Effector Activity: | Muted |
| Synonyms: | Anifrolumab, MEDI-546, IFNAR1, IFNAR, Interferon α/β Receptor 1 |
| Product Concentration: | ≥ 5.0 mg/ml |
| Purity: | ≥95% by SDS Page |
| Immunogen: | Human IFNAR1 |
| Endotoxin Level: | < 1.0 EU/mg as determined by the LAL method |
| Antigen Distribution: | IFNAR1 is a plasma membrane protein widely expressed on most nucleated cells that undergoes endocytosis when activated. |
| Formulation: | This biosimilar antibody is aseptically packaged and formulated in 0.01 M phosphate buffered saline (150 mM NaCl) PBS pH 7.2 - 7.4 with no carrier protein, potassium, calcium or preservatives added. Due to inherent biochemical properties of antibodies, certain products may be prone to precipitation over time. Precipitation may be removed by aseptic centrifugation and/or filtration. |
| Specificity: | This non-therapeutic biosimilar antibody uses the same variable region sequence as the therapeutic antibody Anifrolumab. This product is for research use only. Anifrolumab activity is directed against Human IFNAR1. |
| Additional Applications Reported In Literature: | ELISA WP IP FA FC IHC |
Type I interferon (IFN) receptor (IFNAR) plays a central role in anti-viral and anti-proliferative responses and its endocytic trafficking is tightly associated with control of JAK/STAT signaling1. IFNAR is composed of two subunits, IFNAR1 and IFNAR2, that are ubiquitously expressed at variable levels depending on the cell type. IFNAR1 plays a role in the pathogenesis of complex multisystem autoimmune diseases such as systemic lupus erythematosus (SLE)2 and systemic sclerosis3. Approximately 60-80% of adult patients with active SLE express elevated levels of type I IFN inducible genes in tissues and blood 4, known as an ‘IFN signature'2.
Anifrolumab is an IFNAR1-specific antagonist produced in mouse myeloma cells (NS0)4, 5 that prevents IFN from binding to IFNAR12 and suppresses the receptor-mediated biological activity of all type I IFNs3, including those implicated in SLE pathogenesis (IFN-α, IFN-β and IFN-ω)5. Anifrolumab binding leads to inhibition of downstream signaling activities4, 6, including IFN responsive gene expression2. Anifrolumab also normalizes the IFN gene signature in patients with systemic sclerosis6.
Anifrolumab clone AL 5, a non-therapeutic biosimilar antibody for research use only was developed recombinantly and has the same variable regions as the original therapeutic which binds to IFNAR1 with high specificity and affinity, sterically inhibiting the binding of IFN ligands7 and preventing the formation of the IFN/IFNAR1/IFNAR2 ternary signaling complex by blocking heterodimerization2, 7. Additionally, anifrolumab induces internalization of IFNAR1, reducing the levels of cell surface IFNAR1 available for complex assembly2, 4. Anifrolumab recognizes the SD3 subdomain of IFNAR1 with the critical residue R279 providing a dominant contribution7.
Anifrolumab is an Fc-modified version of the anti-IFNAR 9D4 antibody8. Anifrolumab’s constant domain contains the triple mutations L234F/L235E/P331S for reduced antibody Fc-mediated effector functions7 and causes decreased binding to human FcyRI (CD64), FcyRIIA (CD32A), FcyRIII (CD16), and Clq8.
| Ligand/Receptor: | IFNAR1 |
| State of Matter: | Liquid |
| Regulatory Status: | Research Use Only (RUO). Non-Therapeutic. |
| Product Preparation: | Recombinant biosimilar antibodies are manufactured in an animal free facility using onlyin vitroprotein free cell culture techniques and are purified by a multi-step process including the use of protein A or G to assure extremely low levels of endotoxins, leachable protein A or aggregates. |
| Pathogen Testing: | To protect mouse colonies from infection by pathogens and to assure that experimental preclinical data is not affected by such pathogens, all of Leinco’s recombinant biosimilar antibodies are tested and guaranteed to be negative for all pathogens in the IDEXX IMPACT I Mouse Profile. |
| Storage and Handling: | Functional grade biosimilar antibodies may be stored sterile as received at 2-8°C for up to one month. For longer term storage, aseptically aliquot in working volumes without diluting and store at -80°C. Avoid Repeated Freeze Thaw Cycles. |
| UniProt: | P17181 |
| Research Area: | Biosimilars |