Filgrastim ELISA Kit (Antibody screening) Quantitative
- SKU:
- HUMB00038
- Product Type:
- ELISA Kit
- ELISA Type:
- Biosimilar ELISA
- Biosimilar ELISA Type:
- Quantitative
- Applications:
- ELISA
- Reactivity:
- Human
- Analytes:
- Filgrastim ELISA Kit
- Research Area:
- Immune Stimulation
Description
Anti-Filgrastim ADA Quantitative ELISA Kit
Enzyme-linked immunosorbent assay for the quantitative determination of specific antibodies to Filgrastim in human serum and plasma with confirmation reagent (for 20 samples). The Assay Genie anti-filgrastim ELISA Kit is intended for the quantitative determination of Filgrastim anti-drug antibodies in serum and plasma. It is for professional use only.
Anti-Filgrastim ADA Quantitative ELISA Kit test principle
The Assay Genie Antibody to Filgrastim ELISA is a sandwich assay for the determination of anti-filgrastim antibodies in serum and plasma samples. During the first incubation period, the drug filgrastim coated on the wall of the microtiter wells captures the antibodies to filgrastim in patient serum and plasma samples. After washing away the unbound components from samples, a Peroxidase-labelled conjugate (which detects IgG, IgM, IgA and IgE antibodies) is added to each well and then incubated. After a second washing step, the bound enzymatic activity is detected by addition of tetramethylbenzidine (TMB) chromogen-substrate. Finally, the reaction is terminated with an acidic stop solution. The intensity of the reaction colour is directly proportional to the concentration of anti-filgrastim antibodies in a sample.
Anti-Filgrastim ADA Quantitative ELISA Product Information
| Information | Description |
| Application | Free drug |
| Required Volume (μl) | 10 |
| Total Time (min) | 200 |
| Sample Type | Serum, Plasma |
| Number of Assays | 96 |
| Detection Limit (ng/mL) | 3 (ng/mL) |
| Spike Recovery (%) | 85-115% |
| Shelf Life (year) | 6 Months |
| Alternative Names | Granulocyte Colony Stimulating Factor (G-CSF) |
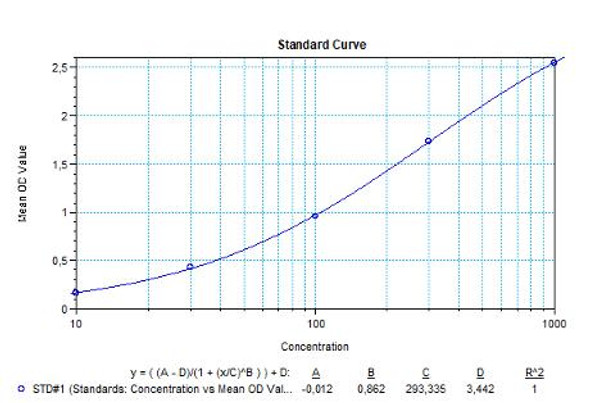
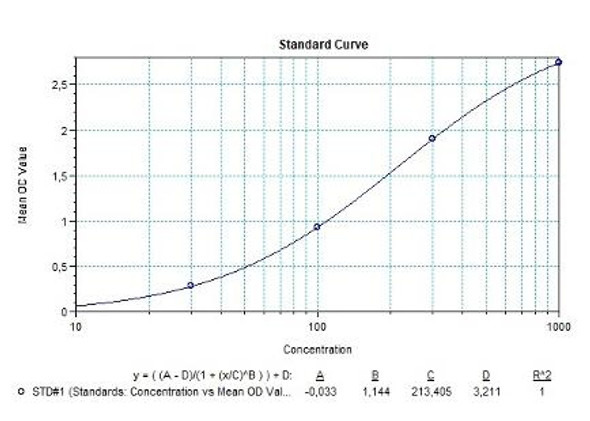
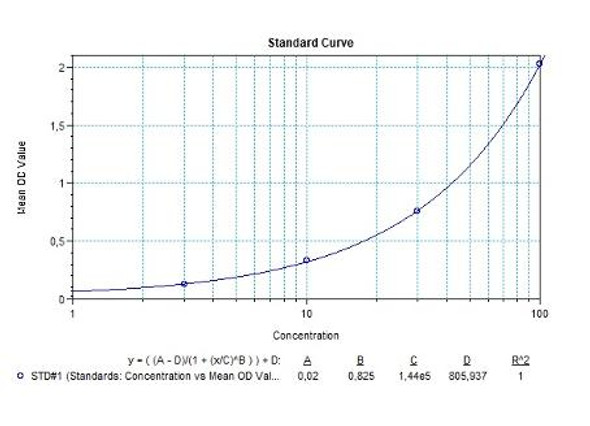
Anti-Filgrastim ADA Quantitative ELISA - Key Information
Filgrastim mode of action
Filgrastim is a recombinant, non-pegylated human granulocyte colony stimulating factor (G-CSF) analogue manufactured by recombinant DNA technology using a strain of E. coli. Chemically, it consists of 175 amino acid residues. The protein has an amino acid sequence that is identical to the natural sequence predicted from human DNA sequence analysis, except for the addition of an N-terminal methionine necessary for expression in E coli. Filgrastim binds to the G-CSF receptor and stimulates the production of neutrophils in the bone marrow. As a GCSF analog, it controls proliferation of committed progenitor cells and influences their maturation into mature neutrophils. Filgrastim also stimulates the release of neutrophils from bone marrow storage pools and reduces their maturation time. Filgrastim acts to increase the phagocytic activity of mature neutrophils.
Filgrastim uses
In patients receiving cytotoxic chemotherapy, Filgrastim can accelerate neutrophil recovery, leading to a reduction in duration of the neutropenic phase. Filgrastim is used in patients with acute myeloid leukemia receiving induction or consolidation chemotherapy. It is also used in cancer patients receiving bone marrow transplant. In general, filgrastim increases neutrophil counts in order to decrease the risk of infection or duration of neutropenia. Neutropenia is an adverse event associated with chemotherapy. Filgrastim is also indicated for patients with severe chronic neutropenia. It mobilizes hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis to allow for a more rapid engraftment.
Filgrastim treatment
Used in the treatment of chemotherapy-induced neutropenia by enhancing the production of neutrophils. Filgrastim acts on hematopoietic cells by binding to specific cell surface receptors thereby stimulating proliferation, differentiation, commitment, and end cell functional activation. When filgrastim was administered to cancer patients, it took 3-5 days to reach maximum absolute neutrophil count (ANC). Levels of neutrophils returned to baseline by 21 days following completion of chemotherapy. In the healthy volunteer trials, doubling the filgrastim subcutaneous dose from 5 to 10 mcg/kg resulted in a 16-19% increase in the ANC max and a 33-36% increase in the area under the effect curve for ANC.
Filgrastim immunogenicity
As with any biologic therapeutic, immunogenicity, in the form of anti-filgrastim antibodies can occur. The demonstration of anti-filgrastim antibodies during treatment with filgrastim is a major concern. The Assay Genie Anti-Filgrastim ADA Quantitative ELISA Kit can be efficiently used for monitoring anti-filgrastim antibodies in biological samples and is for research use only.
Anti-Filgrastim ADA Quantitative ELISA Kit Contents
| Size | Kit Contents |
| 1 x 12 x 8 | Microtiter Plate Break apart strips. Microtiter plate with 12 rows each of 8 wells coated with reactant |
| 8 x 0.3 mL | Filgrastim Standards A-F (10X), High Level Control, Low Level Control 500; 250; 125; 62.5; 31.25; 0 ng/mL |
| 1 x 30 mL | Assay Buffer |
| 1 x 12 mL | Peroxidase Conjugate |
| 1 x 12 mL | TMB Substrate Solution |
| 1 x 12 mL | TMB Stop Solution |
| 1 x 50 mL | Wash Buffer concentrate (20x) |
| 2 x 1 | Adhesive Foil |
| 1 x 210 µL | Confirmation Reagent (For 20 Samples) |
Anti-Filgrastim ADA Quantitative ELISA Protocol
| Steps | Protocol |
| 1 | Dilute each of the standards and samples (serum/plasma) using Assay Buffer as described in |
| 2 | Pipette 100 µL of each Diluted Standards, High Level Control, Low Level Control or diluted Serum Wells |
| 3 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
| 4 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300µL of diluted. Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 5 | Pipette 100 µL of ready-to use Peroxidase Conjugate into each well. |
| 6 | Cover the plate with adhesive foil. Incubate 60 min at room temperature (18- 25°C). |
| 7 | Remove adhesive foil. Discard incubation solution. Wash plate 3 times each with 300 µL of diluted Wash Buffer. Remove excess solution by tapping the inverted plate on a paper towel. |
| 8 | Pipette 100 µL of TMB Substrate Solution into each well. |
| 9 | Incubate 20 min (without adhesive foil) at room temperature (18-25°C) in the dark |
| 10 | Stop the substrate reaction by adding 100 µL of Stop Solution into each well. Briefly mix contents by gently shaking the plate. Colour changes from blue to yellow. |
| 11 | Measure optical density with a photometer at 450/650 nm within 30 min after pipetting of the Stop Solution. |
Trademarks
NEUPOGEN® is the Amgen Inc. trademark for filgrastim