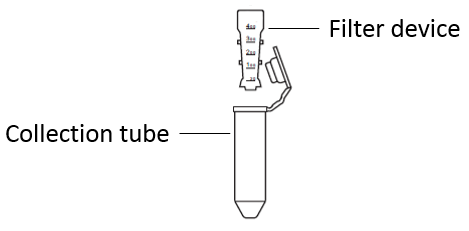
| Concentration & buffer exchange: | Put the filter device in the collection tube, add 1 mg of protein to be labeled into the filter device, add Labeling Buffer II to the final volume of 5 mL, cover the filter tube, centrifuge at 12,000×g for 10 min, and discard the liquid in the collection tube. |
| Note: | The maximum volume of the filter device is 5 mLIf the volume of 1 mg protein is greater than 5 mL, please add it in several times and concentrate it by centrifugation and ultrafiltrationIf the protein to be labeled contains free amino groups (Tris, amino acids or other interferents, repeat ultrafiltration with Labeling Buffer II to ensure that it is removed full. |
| Recovery & quantification: | Invert the filter device into the collection tube, centrifuge at 1000×g for 2 min, collect the protein in the collection tube, take out the filter device, add an appropriate amount of Labeling Buffer II into the collection tube, make sure that the protein concentration is about 2 mg/mL. At the same time, add 5 mL Labeling Buffer II into the filter device and put it on a pipe rack for later use. |
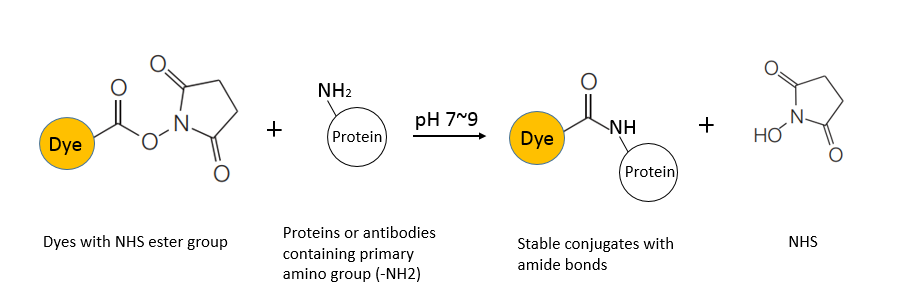
| Labeling reaction: | Immediately add 8 μL of 31 mM AF 647 to the protein solution, gently blow and mix fully, sealed with a lid, and incubate at 37°C for 30 min in the dark. (Optiona |
| Blocking: | Add 1 M Tris (pH 7) to stop the reaction at the ratio of 10 μL of 1 M Tris (pH 7) per 100 μg protein, mix fully and incubate at room temperature for 10 min. |
| Ultrafiltration & purification: | Add an appropriate amount of 1×PBS into the above reaction solution to the final volume of 5 mL, gently mix and transfer the reaction solution to the filter device, make sure that the Labeling Buffer II in the filter device in step 2 should be discard (if the above reaction solution exceeds 5 mL, it can be transferred to the 5 spin-dried filter device for several times after ultrafiltratio, and cover the cap after matching with the collection tube, and centrifuge for 10~30 min at the speed of 12,000×g. Discard the liquid in the collection tube, replenish 1×PBS to 500 μL in the filter device, and repeat the centrifugal ultrafiltration operation for 2~3 times until the color of the ultrafiltrate in the collection tube is almost colorless and transparent. |
| Collect labeled product: | Add 2 mL 1×PBS into the filter device and pipet gently. Invert the filter device in another collection tube and centrifuge at 1000×g for 2 min. Collect the solution in the collection tube, which is the protein labeled by AF (OptionaDetermining the degree of labeling Use an absorbing light scanning device to set the scanning range of 230 nm~800 nm. Set 1×PBS to as blank control. Take 2 μL AF 647 labeled sample, scan the absorption spectrum (230 nm to 800 nand record A280 and A655 data (1 cm optical pat. |
| Note: | In this case, it is necessary to scan the absorption value in the range of 230nm~800nm, rather than measuring the value at A280 and ABy observing the absorption spectrum curve, some abnormal values can be eliminated, such as the measurement error caused by bubbles in the sampleThe DOS and protein concentration can be calculated based on the molar extinction coefficient of AF 647 dye, the A280 correction value, the molar extinction coefficient of the protein, the molecular weight of the protein, etcThe calculation formula is as follows: DOS = (A655×εIgG)/( ε AF 647×(A280-CF280×A655)) Protein concentration (mg/mL)=(A280-CF280×A AF 647)×150000/εIgG 6 |